As filed with the Securities and Exchange Commission on
October 2
7
, 2021 Registration No. 333-
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM S-1
REGISTRATION STATEMENT
UNDER
THE SECURITIES ACT OF 1933
(Exact name of registrant as specified in its charter)
Delaware |
3829 |
83-0974996 | ||
(State or other jurisdiction of incorporation or organization) |
(Primary Standard Industrial Classification Code Number) |
(I.R.S. Employer Identification Number) |
1218 Menlo Drive
Atlanta, Georgia 30318
(470)
870-2700
(Address, including zip code, and telephone number, including area code, of registrant’s principal executive offices)
Thomas D. Logan, Chief Executive Officer
Mirion Technologies, Inc.
1218 Menlo Drive
Atlanta, Georgia 30318
(470)
870-2700
(Name, address, including zip code, and telephone number, including area code, of agent for service)
With copies to:
| Emmanuelle Lee, General Counsel Mirion Technologies, Inc. 1218 Menlo Drive Atlanta, Georgia 30318 (470) 870-2700 |
Alan F. Denenberg Stephen Salmon Bryan M. Quinn Davis Polk and Wardwell LLP 1600 El Camino Real Menlo Park, California 94025 (650) 752-2000 |
Approximate date of commencement of proposed sale to the public:
If any of the securities being registered on this Form are to be offered on a delayed or continuous basis pursuant to Rule 415 under the Securities Act of 1933, check the following box: ☒
If this Form is filed to register additional securities for an offering pursuant to Rule 462(b) under the Securities Act, please check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. ☐
If this Form is a post-effective amendment filed pursuant to Rule 462(c) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. ☐
If this Form is a post-effective amendment filed pursuant to Rule 462(d) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. ☐
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a
non-accelerated
filer, a smaller reporting company, or an emerging growth company. See the definitions of “large accelerated filer,” “accelerated filer,” “smaller reporting company,” and “emerging growth company” in Rule 12b-2
of the Exchange Act. | Large accelerated filer | ☐ | Accelerated filer | ☐ | |||
Non-accelerated filer |
☒ | Smaller reporting company | ||||
| Emerging growth company | ||||||
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided to Section 7(a)(2)(B) of the Securities Act. ☐
CALCULATION OF REGISTRATION FEE
| | ||||||||
Title of Each Class of Securities to be Registered |
Amount to be Registered (1) |
Proposed Maximum Offering Price Per Share |
Proposed Maximum Aggregate Offering Price |
Amount of Registration Fee (2) | ||||
| Primary Offering: |
||||||||
| Class A common stock, $0.0001 par value per share |
27,249,979 (3) |
$11.50 (4) |
$313,374,758.50 |
$29,049.84 | ||||
| Class A common stock, $0.0001 par value per share |
8,560,540 (5) |
$10.68 (6) |
$91,426,567.20 |
$8,475.24 | ||||
| Secondary Offering: |
||||||||
| Class A common stock, $0.0001 par value per share |
152,157,565 (7) |
$10.68 (6) |
$1,625,042,794.20 |
$150,641.47 | ||||
| Total |
$2,029,844,119.90 |
$188,166.55 | ||||||
| | ||||||||
| | ||||||||
(1) |
Pursuant to Rule 416 under the Securities Act of 1933, as amended (the “Securities Act”), the registrant is also registering an indeterminate number of additional securities that may become issuable to prevent dilution as a result of any stock dividend, stock split, recapitalization or other similar transaction. |
(2) |
Calculated by multiplying the proposed maximum aggregate offering price of securities to be registered by 0.0000927. |
(3) |
Consists of the primary issuance of an aggregate of 27,249,979 shares of Class A common stock, par value $0.0001 per share (“Class A common stock”), of Mirion Technologies, Inc. (the “Company”), consisting of (i) 18,749,979 shares of Class A common stock issuable upon the exercise of the public warrants (as defined below) by the holders thereof and (ii) 8,500,000 shares of Class A common stock issuable upon the exercise of the private placement warrants (as defined below) by the holders thereof. |
(4) |
Estimated solely for the purpose of calculating the registration fee pursuant to Rule 457(a) under the Securities Act based on the exercise price of the public warrants and private placement warrants. |
(5) |
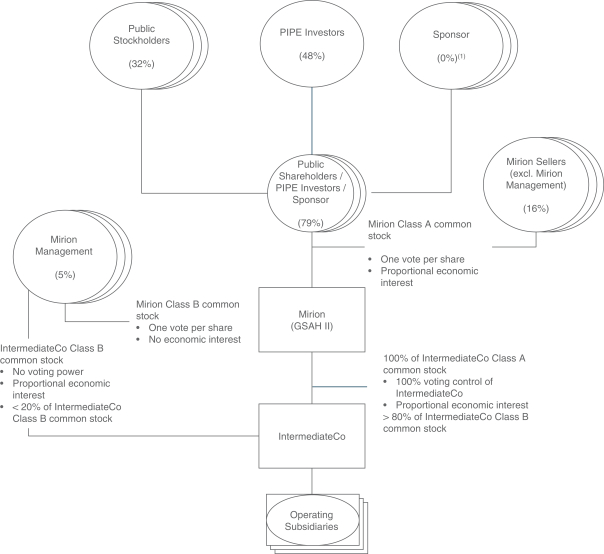
Consists of the primary issuance of 8,560,540 shares of Class A common stock issuable upon the redemption of 8,560,540 shares of Class B common stock, par value $0.0001 per share (the “IntermediateCo Class B common stock”), of Mirion IntermediateCo, Inc. (“IntermediateCo”). |
(6) |
Pursuant to Rule 457(a) under the Securities Act, and solely for the purpose of calculating the registration fee, the proposed maximum offering price is $10.68 per share, which is the average of the high and low prices of shares of the Class A common stock on October 21, 2021 on the New York Stock Exchange (the “NYSE”). |
(7) |
Consists of an aggregate of 152,157,565 shares of Class A common stock registered for resale by the selling holders named in this registration statement (the “Selling Holders”), consisting of (i) 116,347,025 issued and outstanding shares of Class A common stock, (ii) 18,750,000 shares of Class A common stock subject to vesting requirements, (iii) 8,560,540 shares of Class A common stock issuable upon the redemption of 8,560,540 shares of IntermediateCo Class B common stock and (iv) 8,500,000 shares of Class A common stock issuable upon exercise of the private placement warrants. |
The Registrant hereby amends this Registration Statement on such date or dates as may be necessary to delay its effective date until the Registrant shall file a further amendment which specifically states that this Registration Statement shall thereafter become effective in accordance with Section 8(a) of the Securities Act of 1933 or until the Registration Statement shall become effective on such date as the Commission, acting pursuant to said Section 8(a), may determine.
The information in this prospectus is not complete and may be changed. Neither we nor the selling stockholders may sell or distribute the securities described herein until the registration statement filed with the Securities and Exchange Commission is effective. This prospectus is not an offer to sell and is not soliciting an offer to buy the securities in any jurisdiction where the offer or sale is not permitted.
SUBJECT TO COMPLETION, DATED OCTOBER 2
7
, 2021 PRELIMINARY PROSPECTUS

Mirion Technologies, Inc.
Up to 8,560,540 Shares of our Class A Common Stock Issuable upon Redemption of Shares of IntermediateCo Class B Common Stock
Up to 27,249,979 Shares of our Class A Common Stock Issuable upon Exercise of Warrants
152,157,565 Shares of our Class A Common Stock for Resale by the Selling Holders
This prospectus relates to: (1) the issuance by us of up to an aggregate of 35,810,519 shares of Class A common stock, par value $0.0001 per share (“Class A common stock”), of Mirion Technologies, Inc. (the “Company”) that may be issued upon (i) the exercise of 27,249,979 warrants to purchase Class A common stock at an exercise price of $11.50 per share of Class A common stock, including the public warrants and the private placement warrants (each as defined below), and (ii) the exchange of up to 8,560,540 shares of Class B common stock, par value $0.0001 per share (the “IntermediateCo Class B common stock”), of Mirion IntermediateCo, Inc. (“IntermediateCo”); and (2) the offer and sale, from time to time, by the selling holders identified in this prospectus (the “Selling Holders”), or their permitted transferees, of up to 152,157,565 shares of Class A common stock.
This prospectus provides you with a general description of such securities and the general manner in which we and the Selling Holders may offer or sell the securities. More specific terms of any securities that we and the Selling Holders may offer or sell may be provided in a prospectus supplement that describes, among other things, the specific amounts and prices of the securities being offered and the terms of the offering. The prospectus supplement may also add, update or change information contained in this prospectus.
We will not receive any proceeds from the sale of shares of Class A common stock by the Selling Holders pursuant to this prospectus or from the sale of shares of Class A common stock by us pursuant to this prospectus, except with respect to amounts received by us upon exercise of the warrants to the extent such warrants are exercised for cash. However, we will pay the expenses, other than underwriting discounts and commissions, associated with the sale of securities pursuant to this prospectus.
Our registration of the securities covered by this prospectus does not mean that either we or the Selling Holders will issue, offer or sell, as applicable, any of the securities. The Selling Holders may offer and sell the securities covered by this prospectus in a number of different ways and at varying prices. We provide more information in the section entitled “Plan of Distribution.”
You should read this prospectus and any prospectus supplement or amendment carefully before you invest in our securities.
Our Class A common stock and warrants are traded on the New York Stock Exchange (“NYSE”) under the symbols “MIR” and “MIRW,” respectively. On October 26, 2021, the closing price of our Class A common stock was $10.69 per share and the closing price of our warrants was $2.30 per warrant.
We are an “emerging growth company,” as that term is defined under the federal securities laws and, as such, are subject to certain reduced public company reporting requirements.
Investing in our securities involves risks. See “Risk Factors” beginning on page 10 and in any applicable prospectus supplement.
Neither the Securities and Exchange Commission nor any state securities commission has approved or disapproved of the securities or determined if this prospectus is truthful or complete. Any representation to the contrary is a criminal offense.
The date of this prospectus is , 2021.