FYFALSE20210001809987P7YP5YP7YP7YP5Yhttp://fasb.org/us-gaap/2021-01-31#OperatingLeaseRightOfUseAssethttp://fasb.org/us-gaap/2021-01-31#OtherAssetsNoncurrenthttp://fasb.org/us-gaap/2021-01-31#AccruedLiabilitiesCurrenthttp://fasb.org/us-gaap/2021-01-31#OperatingLeaseLiabilityNoncurrenthttp://fasb.org/us-gaap/2021-01-31#AccruedLiabilitiesAndOtherLiabilitieshttp://www.mirion.com/20211231#DeferredIncomeTaxAssetsNetAndOtherNoncurrentLiabilitiesP3Y00018099872021-01-012021-12-310001809987us-gaap:CommonClassAMember2021-01-012021-12-310001809987us-gaap:WarrantMember2021-01-012021-12-3100018099872021-06-30iso4217:USD0001809987us-gaap:CommonClassAMember2022-02-22xbrli:shares0001809987us-gaap:CommonClassBMember2022-02-2200018099872021-12-3100018099872020-06-300001809987us-gaap:CommonClassAMember2021-12-31iso4217:USDxbrli:shares0001809987us-gaap:CommonClassAMember2021-06-300001809987us-gaap:CommonClassAMember2020-06-300001809987us-gaap:CommonClassBMember2021-12-310001809987us-gaap:CommonClassBMember2021-06-300001809987us-gaap:CommonClassBMember2020-06-300001809987mir:AOrdinarySharesMember2021-06-300001809987mir:AOrdinarySharesMember2020-06-300001809987mir:AOrdinarySharesMember2021-12-310001809987mir:BOrdinarySharesMember2021-06-300001809987mir:BOrdinarySharesMember2020-06-300001809987mir:BOrdinarySharesMember2021-12-310001809987us-gaap:ProductMember2021-10-202021-12-310001809987us-gaap:ProductMember2021-07-012021-10-190001809987us-gaap:ProductMember2020-07-012021-06-300001809987us-gaap:ProductMember2019-07-012020-06-300001809987us-gaap:ProductMember2018-07-012019-06-300001809987us-gaap:ServiceMember2021-10-202021-12-310001809987us-gaap:ServiceMember2021-07-012021-10-190001809987us-gaap:ServiceMember2020-07-012021-06-300001809987us-gaap:ServiceMember2019-07-012020-06-300001809987us-gaap:ServiceMember2018-07-012019-06-3000018099872021-10-202021-12-3100018099872021-07-012021-10-1900018099872020-07-012021-06-3000018099872019-07-012020-06-3000018099872018-07-012019-06-300001809987mir:OrdinarySharesMembermir:AOrdinarySharesMember2018-06-300001809987mir:OrdinarySharesMembermir:BOrdinarySharesMember2018-06-300001809987us-gaap:AdditionalPaidInCapitalMember2018-06-300001809987mir:ReceivableFromEmployeesForIssuanceOfCapitalStockMember2018-06-300001809987us-gaap:RetainedEarningsMember2018-06-300001809987us-gaap:AccumulatedOtherComprehensiveIncomeMember2018-06-300001809987us-gaap:NoncontrollingInterestMember2018-06-3000018099872018-06-300001809987us-gaap:NoncontrollingInterestMember2018-07-012019-06-300001809987us-gaap:AdditionalPaidInCapitalMember2018-07-012019-06-300001809987mir:ReceivableFromEmployeesForIssuanceOfCapitalStockMember2018-07-012019-06-300001809987us-gaap:RetainedEarningsMember2018-07-012019-06-300001809987us-gaap:AccumulatedOtherComprehensiveIncomeMember2018-07-012019-06-300001809987mir:OrdinarySharesMembermir:AOrdinarySharesMember2019-06-300001809987mir:OrdinarySharesMembermir:BOrdinarySharesMember2019-06-300001809987us-gaap:AdditionalPaidInCapitalMember2019-06-300001809987mir:ReceivableFromEmployeesForIssuanceOfCapitalStockMember2019-06-300001809987us-gaap:RetainedEarningsMember2019-06-300001809987us-gaap:AccumulatedOtherComprehensiveIncomeMember2019-06-300001809987us-gaap:NoncontrollingInterestMember2019-06-3000018099872019-06-300001809987us-gaap:NoncontrollingInterestMember2019-07-012020-06-300001809987us-gaap:AdditionalPaidInCapitalMember2019-07-012020-06-300001809987mir:ReceivableFromEmployeesForIssuanceOfCapitalStockMember2019-07-012020-06-300001809987us-gaap:RetainedEarningsMember2019-07-012020-06-300001809987us-gaap:AccumulatedOtherComprehensiveIncomeMember2019-07-012020-06-300001809987mir:OrdinarySharesMembermir:AOrdinarySharesMember2020-06-300001809987mir:OrdinarySharesMembermir:BOrdinarySharesMember2020-06-300001809987us-gaap:AdditionalPaidInCapitalMember2020-06-300001809987mir:ReceivableFromEmployeesForIssuanceOfCapitalStockMember2020-06-300001809987us-gaap:RetainedEarningsMember2020-06-300001809987us-gaap:AccumulatedOtherComprehensiveIncomeMember2020-06-300001809987us-gaap:NoncontrollingInterestMember2020-06-300001809987mir:ReceivableFromEmployeesForIssuanceOfCapitalStockMember2020-07-012021-06-300001809987us-gaap:RetainedEarningsMember2020-07-012021-06-300001809987us-gaap:NoncontrollingInterestMember2020-07-012021-06-300001809987us-gaap:AccumulatedOtherComprehensiveIncomeMember2020-07-012021-06-300001809987mir:OrdinarySharesMembermir:AOrdinarySharesMember2021-06-300001809987mir:OrdinarySharesMembermir:BOrdinarySharesMember2021-06-300001809987us-gaap:AdditionalPaidInCapitalMember2021-06-300001809987mir:ReceivableFromEmployeesForIssuanceOfCapitalStockMember2021-06-300001809987us-gaap:RetainedEarningsMember2021-06-300001809987us-gaap:AccumulatedOtherComprehensiveIncomeMember2021-06-300001809987us-gaap:NoncontrollingInterestMember2021-06-300001809987us-gaap:AdditionalPaidInCapitalMember2021-07-012021-10-190001809987srt:CumulativeEffectPeriodOfAdoptionAdjustmentMemberus-gaap:RetainedEarningsMemberus-gaap:AccountingStandardsUpdate201602Member2021-06-300001809987srt:CumulativeEffectPeriodOfAdoptionAdjustmentMemberus-gaap:AccountingStandardsUpdate201602Member2021-06-300001809987mir:ReceivableFromEmployeesForIssuanceOfCapitalStockMember2021-07-012021-10-190001809987us-gaap:RetainedEarningsMember2021-07-012021-10-190001809987us-gaap:AccumulatedOtherComprehensiveIncomeMember2021-07-012021-10-190001809987mir:OrdinarySharesMembermir:AOrdinarySharesMember2021-10-190001809987mir:OrdinarySharesMembermir:BOrdinarySharesMember2021-10-190001809987us-gaap:AdditionalPaidInCapitalMember2021-10-190001809987mir:ReceivableFromEmployeesForIssuanceOfCapitalStockMember2021-10-190001809987us-gaap:RetainedEarningsMember2021-10-190001809987us-gaap:AccumulatedOtherComprehensiveIncomeMember2021-10-190001809987us-gaap:NoncontrollingInterestMember2021-10-1900018099872021-10-190001809987us-gaap:CommonClassBMember2021-10-200001809987us-gaap:RetainedEarningsMember2021-10-2000018099872021-10-200001809987us-gaap:CommonClassAMember2021-10-202021-12-310001809987us-gaap:CommonClassBMember2021-10-202021-12-310001809987us-gaap:AdditionalPaidInCapitalMember2021-10-202021-12-310001809987us-gaap:PrivatePlacementMemberus-gaap:CommonClassAMember2021-10-202021-12-310001809987us-gaap:AdditionalPaidInCapitalMemberus-gaap:PrivatePlacementMember2021-10-202021-12-310001809987us-gaap:PrivatePlacementMember2021-10-202021-12-310001809987us-gaap:NoncontrollingInterestMember2021-10-202021-12-310001809987us-gaap:RetainedEarningsMember2021-10-202021-12-310001809987us-gaap:AccumulatedOtherComprehensiveIncomeMember2021-10-202021-12-310001809987us-gaap:AdditionalPaidInCapitalMember2021-12-310001809987us-gaap:RetainedEarningsMember2021-12-310001809987us-gaap:AccumulatedOtherComprehensiveIncomeMember2021-12-310001809987us-gaap:NoncontrollingInterestMember2021-12-3100018099872021-10-202021-10-200001809987mir:GsahMember2021-10-1900018099872021-07-0100018099872020-07-0100018099872019-07-0100018099872018-07-01mir:Segment0001809987us-gaap:CommonClassAMember2021-10-202021-10-200001809987us-gaap:CommonClassAMember2021-10-20xbrli:pure0001809987mir:IntermediateCoMemberus-gaap:CommonClassAMember2021-10-202021-10-200001809987mir:IntermediateCoMemberus-gaap:CommonClassBMember2021-10-202021-10-200001809987srt:MinimumMemberus-gaap:CustomerRelatedIntangibleAssetsMember2021-01-012021-12-310001809987srt:MaximumMemberus-gaap:CustomerRelatedIntangibleAssetsMember2021-01-012021-12-310001809987srt:MinimumMemberus-gaap:DevelopedTechnologyRightsMember2021-01-012021-12-310001809987srt:MaximumMemberus-gaap:DevelopedTechnologyRightsMember2021-01-012021-12-310001809987srt:MinimumMemberus-gaap:TradeNamesMember2021-01-012021-12-310001809987srt:MaximumMemberus-gaap:TradeNamesMember2021-01-012021-12-310001809987mir:DeferredIncomeTaxesAndOtherLiabilitiesMember2021-12-310001809987mir:DeferredIncomeTaxesAndOtherLiabilitiesMember2021-06-300001809987mir:DeferredIncomeTaxesAndOtherLiabilitiesMember2020-06-300001809987srt:MinimumMember2020-07-012021-06-300001809987srt:MaximumMember2020-07-012021-06-300001809987srt:ScenarioForecastMember2022-06-300001809987srt:ScenarioForecastMember2023-06-300001809987srt:ScenarioForecastMember2024-06-300001809987us-gaap:SalesRevenueNetMemberus-gaap:RevenueFromRightsConcentrationRiskMember2019-07-012020-06-300001809987us-gaap:SalesRevenueNetMemberus-gaap:RevenueFromRightsConcentrationRiskMember2020-07-012021-06-300001809987us-gaap:SalesRevenueNetMemberus-gaap:RevenueFromRightsConcentrationRiskMember2018-07-012019-06-300001809987mir:InterestRateSwapCapAgreementMember2017-07-010001809987mir:InterestRateSwapCapAgreementMember2018-12-310001809987mir:InterestRateSwapCapAgreementMember2020-03-3100018099872020-03-310001809987mir:InterestRateSwapCapAgreementMember2021-06-300001809987mir:InterestRateSwapCapAgreementMember2021-12-310001809987mir:InterestRateSwapCapAgreementMember2019-07-012020-06-300001809987mir:InterestRateSwapCapAgreementMember2018-07-012019-06-300001809987mir:InterestRateSwapCapAgreementMember2021-10-202021-12-310001809987mir:InterestRateSwapCapAgreementMember2020-07-012021-06-300001809987us-gaap:AccountsReceivableMemberus-gaap:RevenueFromRightsConcentrationRiskMember2021-01-012021-12-310001809987us-gaap:AccountsReceivableMemberus-gaap:RevenueFromRightsConcentrationRiskMember2020-07-012021-06-300001809987us-gaap:AccountsReceivableMemberus-gaap:RevenueFromRightsConcentrationRiskMember2019-07-012020-06-300001809987us-gaap:ShareBasedCompensationAwardTrancheOneMember2021-01-012021-12-310001809987us-gaap:ShareBasedCompensationAwardTrancheTwoMember2021-01-012021-12-310001809987us-gaap:ShareBasedCompensationAwardTrancheThreeMember2021-01-012021-12-310001809987us-gaap:RetainedEarningsMember2021-07-012021-07-010001809987mir:IntermediateCoMember2021-10-202021-10-200001809987mir:IntermediateCoMember2021-10-200001809987mir:GsahMember2021-10-202021-10-200001809987mir:MirionMember2021-10-202021-10-200001809987mir:MedicalSegmentMembermir:MirionMember2021-10-202021-10-200001809987mir:IndustrialSegmentMembermir:MirionMember2021-10-202021-10-200001809987us-gaap:CorporateAndOtherMembermir:MirionMember2021-10-202021-10-200001809987mir:MedicalSegmentMembermir:MirionMember2021-10-200001809987mir:IndustrialSegmentMembermir:MirionMember2021-10-200001809987us-gaap:CorporateAndOtherMembermir:MirionMember2021-10-200001809987mir:MirionMember2021-10-200001809987mir:MedicalSegmentMembermir:MirionMemberus-gaap:CustomerRelationshipsMember2021-10-200001809987mir:IndustrialSegmentMembermir:MirionMemberus-gaap:CustomerRelationshipsMember2021-10-200001809987us-gaap:CorporateAndOtherMembermir:MirionMemberus-gaap:CustomerRelationshipsMember2021-10-200001809987us-gaap:CustomerRelationshipsMembermir:MirionMember2021-10-200001809987us-gaap:DevelopedTechnologyRightsMembermir:MedicalSegmentMembermir:MirionMember2021-10-200001809987mir:IndustrialSegmentMemberus-gaap:DevelopedTechnologyRightsMembermir:MirionMember2021-10-200001809987us-gaap:CorporateAndOtherMemberus-gaap:DevelopedTechnologyRightsMembermir:MirionMember2021-10-200001809987us-gaap:DevelopedTechnologyRightsMembermir:MirionMember2021-10-200001809987us-gaap:TradeNamesMembermir:MedicalSegmentMembermir:MirionMember2021-10-200001809987mir:IndustrialSegmentMemberus-gaap:TradeNamesMembermir:MirionMember2021-10-200001809987us-gaap:CorporateAndOtherMemberus-gaap:TradeNamesMembermir:MirionMember2021-10-200001809987us-gaap:TradeNamesMembermir:MirionMember2021-10-200001809987mir:DistributorRelationshipsMembermir:MedicalSegmentMembermir:MirionMember2021-10-200001809987mir:DistributorRelationshipsMembermir:IndustrialSegmentMembermir:MirionMember2021-10-200001809987us-gaap:CorporateAndOtherMembermir:DistributorRelationshipsMembermir:MirionMember2021-10-200001809987mir:DistributorRelationshipsMembermir:MirionMember2021-10-200001809987mir:BacklogAndOtherMembermir:MedicalSegmentMembermir:MirionMember2021-10-200001809987mir:BacklogAndOtherMembermir:IndustrialSegmentMembermir:MirionMember2021-10-200001809987us-gaap:CorporateAndOtherMembermir:BacklogAndOtherMembermir:MirionMember2021-10-200001809987mir:BacklogAndOtherMembermir:MirionMember2021-10-200001809987us-gaap:NoncompeteAgreementsMembermir:MedicalSegmentMembermir:MirionMember2021-10-200001809987mir:IndustrialSegmentMemberus-gaap:NoncompeteAgreementsMembermir:MirionMember2021-10-200001809987us-gaap:CorporateAndOtherMemberus-gaap:NoncompeteAgreementsMembermir:MirionMember2021-10-200001809987us-gaap:NoncompeteAgreementsMembermir:MirionMember2021-10-200001809987srt:MinimumMemberus-gaap:CustomerRelationshipsMember2021-10-202021-10-200001809987srt:MaximumMemberus-gaap:CustomerRelationshipsMember2021-10-202021-10-200001809987srt:MinimumMemberus-gaap:DevelopedTechnologyRightsMember2021-10-202021-10-200001809987srt:MaximumMemberus-gaap:DevelopedTechnologyRightsMember2021-10-202021-10-200001809987us-gaap:TradeNamesMember2021-10-202021-10-200001809987mir:DistributorRelationshipsMembersrt:MinimumMember2021-10-202021-10-200001809987srt:MaximumMembermir:DistributorRelationshipsMember2021-10-202021-10-200001809987mir:BacklogAndOtherMembersrt:MinimumMember2021-10-202021-10-200001809987srt:MaximumMembermir:BacklogAndOtherMember2021-10-202021-10-200001809987us-gaap:NoncompeteAgreementsMember2021-10-202021-10-200001809987mir:MirionMember2021-10-202021-12-310001809987mir:MirionMember2021-07-012021-10-190001809987mir:MirionMember2020-07-012021-06-300001809987us-gaap:FairValueAdjustmentToInventoryMembermir:MirionMember2021-10-202021-12-310001809987us-gaap:FairValueAdjustmentToInventoryMembermir:MirionMember2021-07-012021-10-190001809987us-gaap:FairValueAdjustmentToInventoryMembermir:MirionMember2020-07-012021-06-300001809987us-gaap:AcquisitionRelatedCostsMembermir:MirionMember2021-07-012021-10-190001809987us-gaap:AcquisitionRelatedCostsMembermir:MirionMember2021-10-202021-12-310001809987us-gaap:AcquisitionRelatedCostsMembermir:MirionMember2020-07-012021-06-300001809987mir:CIRSMember2021-12-010001809987mir:SafelineMember2021-12-010001809987mir:SafelineMember2021-12-012021-12-010001809987mir:CHPBadgeMember2021-11-010001809987mir:CHPBadgeMember2021-11-012021-11-010001809987mir:MedicalSegmentMembermir:CIRSMember2021-12-012021-12-010001809987mir:MedicalSegmentMembermir:CIRSMember2021-12-010001809987us-gaap:DevelopedTechnologyRightsMembermir:MedicalSegmentMembermir:CIRSMember2021-12-010001809987mir:MedicalSegmentMembermir:CIRSMemberus-gaap:CustomerRelationshipsMember2021-12-010001809987us-gaap:TradeNamesMembermir:MedicalSegmentMembermir:CIRSMember2021-12-010001809987mir:BacklogAndOtherMembermir:MedicalSegmentMembermir:CIRSMember2021-12-010001809987mir:InterestRateSwapCapAgreementMember2021-12-010001809987us-gaap:DevelopedTechnologyRightsMembermir:CIRSMember2021-12-012021-12-010001809987us-gaap:CustomerRelationshipsMembermir:CIRSMember2021-12-012021-12-010001809987us-gaap:TradeNamesMembermir:CIRSMember2021-12-012021-12-010001809987mir:BacklogAndOtherMembermir:CIRSMember2021-12-012021-12-010001809987mir:MedicalSegmentMembermir:CIRSMember2021-12-310001809987mir:DoverMember2021-09-010001809987mir:DoverMember2021-09-012021-09-010001809987mir:SunnuclearMember2020-12-180001809987mir:SunnuclearMember2020-12-182020-12-180001809987mir:DosimetricsMember2020-12-010001809987mir:DosimetricsMember2020-12-012020-12-010001809987mir:BiodexMember2020-09-010001809987mir:BiodexMember2020-09-012020-09-010001809987mir:AwstMember2020-03-310001809987mir:AwstMember2020-03-312020-03-31iso4217:EUR0001809987mir:SelmicMember2019-10-310001809987mir:SelmicMember2019-10-312019-10-310001809987mir:PremiumAnalyseMember2019-07-190001809987mir:PremiumAnalyseMember2019-07-192019-07-190001809987mir:CapintecMember2019-07-090001809987mir:CapintecMember2019-07-092019-07-090001809987mir:NrgDosimetryServicesGroupMember2018-10-310001809987mir:NrgDosimetryServicesGroupMember2018-10-312018-10-310001809987mir:BiodexMembermir:MedicalSegmentMember2020-07-012021-06-300001809987mir:SunnuclearMembermir:MedicalSegmentMember2020-07-012021-06-300001809987mir:BiodexMembermir:MedicalSegmentMember2021-06-300001809987mir:SunnuclearMembermir:MedicalSegmentMember2021-06-300001809987mir:BiodexMembermir:MedicalSegmentMemberus-gaap:CustomerRelationshipsMember2021-06-300001809987mir:SunnuclearMembermir:MedicalSegmentMemberus-gaap:CustomerRelationshipsMember2021-06-300001809987mir:BiodexMemberus-gaap:TradeNamesMembermir:MedicalSegmentMember2021-06-300001809987us-gaap:TradeNamesMembermir:SunnuclearMembermir:MedicalSegmentMember2021-06-300001809987mir:BiodexMemberus-gaap:NoncompeteAgreementsMembermir:MedicalSegmentMember2021-06-300001809987mir:SunnuclearMemberus-gaap:NoncompeteAgreementsMembermir:MedicalSegmentMember2021-06-300001809987mir:BiodexMemberus-gaap:DevelopedTechnologyRightsMembermir:MedicalSegmentMember2021-06-300001809987us-gaap:DevelopedTechnologyRightsMembermir:SunnuclearMembermir:MedicalSegmentMember2021-06-300001809987srt:MinimumMemberus-gaap:CustomerRelationshipsMember2020-09-012020-09-010001809987srt:MinimumMemberus-gaap:CustomerRelationshipsMember2020-12-182020-12-180001809987srt:MaximumMemberus-gaap:CustomerRelationshipsMember2020-12-182020-12-180001809987srt:MaximumMemberus-gaap:CustomerRelationshipsMember2020-09-012020-09-010001809987us-gaap:TradeNamesMember2020-12-182020-12-180001809987us-gaap:TradeNamesMember2020-09-012020-09-010001809987srt:MinimumMemberus-gaap:NoncompeteAgreementsMember2020-09-012020-09-010001809987srt:MinimumMemberus-gaap:NoncompeteAgreementsMember2020-12-182020-12-180001809987srt:MaximumMemberus-gaap:NoncompeteAgreementsMember2020-12-182020-12-180001809987srt:MaximumMemberus-gaap:NoncompeteAgreementsMember2020-09-012020-09-010001809987srt:MinimumMemberus-gaap:DevelopedTechnologyRightsMember2020-12-182020-12-180001809987srt:MinimumMemberus-gaap:DevelopedTechnologyRightsMember2020-09-012020-09-010001809987srt:MaximumMemberus-gaap:DevelopedTechnologyRightsMember2020-12-182020-12-180001809987srt:MaximumMemberus-gaap:DevelopedTechnologyRightsMember2020-09-012020-09-010001809987mir:SunnuclearMembermir:MedicalSegmentMember2021-02-012021-02-280001809987mir:BiodexAndSNCMember2020-07-012021-06-300001809987mir:BiodexAndSNCMember2019-07-012020-06-300001809987us-gaap:FairValueAdjustmentToInventoryMembermir:BiodexAndSNCMember2019-07-012020-06-300001809987us-gaap:FairValueAdjustmentToInventoryMembermir:BiodexAndSNCMember2020-07-012021-06-300001809987us-gaap:AcquisitionRelatedCostsMembermir:BiodexAndSNCMember2019-07-012020-06-300001809987us-gaap:AcquisitionRelatedCostsMembermir:BiodexAndSNCMember2020-07-012021-06-300001809987mir:BiodexAndSNCMembermir:ReductionInRevenuesDueToTheEliminationOfDeferredContractRevenueMember2020-07-012021-06-300001809987mir:BiodexAndSNCMembermir:ReductionInRevenuesDueToTheEliminationOfDeferredContractRevenueMember2019-07-012020-06-300001809987srt:MinimumMemberus-gaap:LandBuildingsAndImprovementsMember2021-01-012021-12-310001809987srt:MaximumMemberus-gaap:LandBuildingsAndImprovementsMember2021-01-012021-12-310001809987us-gaap:LandBuildingsAndImprovementsMember2021-12-310001809987us-gaap:LandBuildingsAndImprovementsMember2021-06-300001809987us-gaap:LandBuildingsAndImprovementsMember2020-06-300001809987us-gaap:MachineryAndEquipmentMembersrt:MinimumMember2021-01-012021-12-310001809987us-gaap:MachineryAndEquipmentMembersrt:MaximumMember2021-01-012021-12-310001809987us-gaap:MachineryAndEquipmentMember2021-12-310001809987us-gaap:MachineryAndEquipmentMember2021-06-300001809987us-gaap:MachineryAndEquipmentMember2020-06-300001809987mir:BadgesMembersrt:MinimumMember2021-01-012021-12-310001809987mir:BadgesMembersrt:MaximumMember2021-01-012021-12-310001809987mir:BadgesMember2021-12-310001809987mir:BadgesMember2021-06-300001809987mir:BadgesMember2020-06-300001809987srt:MinimumMembermir:FurnitureFixturesComputerEquipmentAndOtherMember2021-01-012021-12-310001809987srt:MaximumMembermir:FurnitureFixturesComputerEquipmentAndOtherMember2021-01-012021-12-310001809987mir:FurnitureFixturesComputerEquipmentAndOtherMember2021-12-310001809987mir:FurnitureFixturesComputerEquipmentAndOtherMember2021-06-300001809987mir:FurnitureFixturesComputerEquipmentAndOtherMember2020-06-300001809987us-gaap:ConstructionInProgressMember2021-12-310001809987us-gaap:ConstructionInProgressMember2021-06-300001809987us-gaap:ConstructionInProgressMember2020-06-300001809987us-gaap:CostOfSalesMember2021-12-310001809987us-gaap:CostOfSalesMember2021-10-190001809987us-gaap:CostOfSalesMember2021-06-300001809987us-gaap:CostOfSalesMember2020-06-300001809987us-gaap:CostOfSalesMember2019-06-300001809987us-gaap:OperatingExpenseMember2021-12-310001809987us-gaap:OperatingExpenseMember2021-10-190001809987us-gaap:OperatingExpenseMember2021-06-300001809987us-gaap:OperatingExpenseMember2020-06-300001809987us-gaap:OperatingExpenseMember2019-06-3000018099872021-07-012021-12-310001809987mir:MedicalMember2019-06-300001809987mir:IndustrialMember2019-06-300001809987mir:MedicalMembermir:CapintecMember2019-07-012020-06-300001809987mir:CapintecMembermir:IndustrialMember2019-07-012020-06-300001809987mir:CapintecMember2019-07-012020-06-300001809987mir:MedicalMembermir:PremiumAnalyseMember2019-07-012020-06-300001809987mir:IndustrialMembermir:PremiumAnalyseMember2019-07-012020-06-300001809987mir:PremiumAnalyseMember2019-07-012020-06-300001809987mir:MedicalMembermir:SelmicMember2019-07-012020-06-300001809987mir:SelmicMembermir:IndustrialMember2019-07-012020-06-300001809987mir:SelmicMember2019-07-012020-06-300001809987mir:MedicalMembermir:AwstMember2019-07-012020-06-300001809987mir:IndustrialMembermir:AwstMember2019-07-012020-06-300001809987mir:AwstMember2019-07-012020-06-300001809987mir:MedicalMember2019-07-012020-06-300001809987mir:IndustrialMember2019-07-012020-06-300001809987mir:MedicalMember2020-06-300001809987mir:IndustrialMember2020-06-300001809987mir:MedicalMembermir:SunnuclearMember2020-07-012021-06-300001809987mir:IndustrialMembermir:SunnuclearMember2020-07-012021-06-300001809987mir:SunnuclearMember2020-07-012021-06-300001809987mir:MedicalMembermir:BiodexMember2020-07-012021-06-300001809987mir:BiodexMembermir:IndustrialMember2020-07-012021-06-300001809987mir:BiodexMember2020-07-012021-06-300001809987mir:MedicalMembermir:DosimetricsMember2020-07-012021-06-300001809987mir:IndustrialMembermir:DosimetricsMember2020-07-012021-06-300001809987mir:DosimetricsMember2020-07-012021-06-300001809987mir:MedicalMember2020-07-012021-06-300001809987mir:IndustrialMember2020-07-012021-06-300001809987mir:MedicalMember2021-06-300001809987mir:IndustrialMember2021-06-300001809987mir:MedicalMembermir:DosimetryBadgeMember2021-07-012021-10-190001809987mir:DosimetryBadgeMembermir:IndustrialMember2021-07-012021-10-190001809987mir:DosimetryBadgeMember2021-07-012021-10-190001809987mir:MedicalMember2021-07-012021-10-190001809987mir:IndustrialMember2021-07-012021-10-190001809987mir:MedicalMember2021-10-190001809987mir:IndustrialMember2021-10-190001809987mir:MedicalMember2021-10-200001809987mir:IndustrialMember2021-10-200001809987mir:MedicalMembermir:MirionMember2021-10-202021-12-310001809987mir:IndustrialMembermir:MirionMember2021-10-202021-12-310001809987mir:MedicalMembermir:CHPBadgeMember2021-10-202021-12-310001809987mir:IndustrialMembermir:CHPBadgeMember2021-10-202021-12-310001809987mir:CHPBadgeMember2021-10-202021-12-310001809987mir:MedicalMembermir:SafelineMember2021-10-202021-12-310001809987mir:IndustrialMembermir:SafelineMember2021-10-202021-12-310001809987mir:SafelineMember2021-10-202021-12-310001809987mir:MedicalMembermir:CIRSMember2021-10-202021-12-310001809987mir:IndustrialMembermir:CIRSMember2021-10-202021-12-310001809987mir:CIRSMember2021-10-202021-12-310001809987mir:MedicalMember2021-10-202021-12-310001809987mir:IndustrialMember2021-10-202021-12-310001809987mir:MedicalMember2021-12-310001809987mir:IndustrialMember2021-12-310001809987srt:MinimumMemberus-gaap:CustomerRelationshipsMember2021-01-012021-12-310001809987srt:MaximumMemberus-gaap:CustomerRelationshipsMember2021-01-012021-12-310001809987us-gaap:CustomerRelationshipsMember2021-12-310001809987mir:DistributorRelationshipsMembersrt:MinimumMember2021-01-012021-12-310001809987srt:MaximumMembermir:DistributorRelationshipsMember2021-01-012021-12-310001809987mir:DistributorRelationshipsMember2021-12-310001809987us-gaap:DevelopedTechnologyRightsMember2021-12-310001809987us-gaap:TradeNamesMember2021-12-310001809987mir:BacklogAndOtherMembersrt:MinimumMember2021-01-012021-12-310001809987srt:MaximumMembermir:BacklogAndOtherMember2021-01-012021-12-310001809987mir:BacklogAndOtherMember2021-12-310001809987srt:MinimumMemberus-gaap:CustomerRelationshipsMember2020-07-012021-06-300001809987srt:MaximumMemberus-gaap:CustomerRelationshipsMember2020-07-012021-06-300001809987us-gaap:CustomerRelationshipsMember2021-06-300001809987mir:DistributorRelationshipsMembersrt:MinimumMember2020-07-012021-06-300001809987srt:MaximumMembermir:DistributorRelationshipsMember2020-07-012021-06-300001809987us-gaap:DevelopedTechnologyRightsMember2021-06-300001809987srt:MinimumMemberus-gaap:TradeNamesMember2020-07-012021-06-300001809987srt:MaximumMemberus-gaap:TradeNamesMember2020-07-012021-06-300001809987us-gaap:TradeNamesMember2021-06-300001809987mir:BacklogAndOtherMembersrt:MinimumMember2020-07-012021-06-300001809987srt:MaximumMembermir:BacklogAndOtherMember2020-07-012021-06-300001809987mir:BacklogAndOtherMember2021-06-300001809987srt:MinimumMemberus-gaap:CustomerRelationshipsMember2019-07-012020-06-300001809987srt:MaximumMemberus-gaap:CustomerRelationshipsMember2019-07-012020-06-300001809987us-gaap:CustomerRelationshipsMember2020-06-300001809987srt:MinimumMemberus-gaap:DevelopedTechnologyRightsMember2019-07-012020-06-300001809987srt:MaximumMemberus-gaap:DevelopedTechnologyRightsMember2019-07-012020-06-300001809987us-gaap:DevelopedTechnologyRightsMember2020-06-300001809987srt:MinimumMemberus-gaap:TradeNamesMember2019-07-012020-06-300001809987srt:MaximumMemberus-gaap:TradeNamesMember2019-07-012020-06-300001809987us-gaap:TradeNamesMember2020-06-300001809987mir:BacklogAndOtherMembersrt:MinimumMember2019-07-012020-06-300001809987srt:MaximumMembermir:BacklogAndOtherMember2019-07-012020-06-300001809987mir:BacklogAndOtherMember2020-06-300001809987us-gaap:CostOfSalesMember2021-10-202021-12-310001809987us-gaap:CostOfSalesMember2021-07-012021-10-190001809987us-gaap:CostOfSalesMember2020-07-012021-06-300001809987us-gaap:CostOfSalesMember2019-07-012020-06-300001809987us-gaap:CostOfSalesMember2018-07-012019-06-300001809987us-gaap:OperatingExpenseMember2021-10-202021-12-310001809987us-gaap:OperatingExpenseMember2021-07-012021-10-190001809987us-gaap:OperatingExpenseMember2020-07-012021-06-300001809987us-gaap:OperatingExpenseMember2019-07-012020-06-300001809987us-gaap:OperatingExpenseMember2018-07-012019-06-300001809987mir:GsahMemberus-gaap:CommonClassAMember2021-10-202021-10-200001809987us-gaap:CommonClassBMembermir:GsahMember2021-10-202021-10-200001809987mir:GsahMember2021-10-200001809987mir:TwoThousandAndTwentyOneCreditAgreementMember2021-12-310001809987mir:TwoThousandAndTwentyOneCreditAgreementMember2021-06-300001809987mir:TwoThousandAndTwentyOneCreditAgreementMember2020-06-300001809987mir:TwoThousandAndNineteenCreditFacilityFirstLienTermLoanMember2021-12-310001809987mir:TwoThousandAndNineteenCreditFacilityFirstLienTermLoanMember2021-06-300001809987mir:TwoThousandAndNineteenCreditFacilityFirstLienTermLoanMember2020-06-300001809987mir:NrgLoanMember2021-12-310001809987mir:NrgLoanMember2021-06-300001809987mir:NrgLoanMember2020-06-300001809987mir:JlgNotePayableMember2021-12-310001809987mir:JlgNotePayableMember2021-06-300001809987mir:JlgNotePayableMember2020-06-300001809987mir:CanadianFinancialInstitutionMember2021-12-310001809987mir:CanadianFinancialInstitutionMember2021-06-300001809987mir:CanadianFinancialInstitutionMember2020-06-300001809987us-gaap:NotesPayableOtherPayablesMember2021-12-310001809987us-gaap:NotesPayableOtherPayablesMember2021-06-300001809987us-gaap:NotesPayableOtherPayablesMember2020-06-300001809987mir:DrawOnRevolvingLineOfCreditMember2021-12-310001809987mir:DrawOnRevolvingLineOfCreditMember2021-06-300001809987mir:DrawOnRevolvingLineOfCreditMember2020-06-300001809987mir:TwoThousandAndTwentyOneCreditAgreementMemberus-gaap:SecuredDebtMember2021-12-310001809987us-gaap:RevolvingCreditFacilityMembermir:TwoThousandAndTwentyOneCreditAgreementMember2021-12-310001809987us-gaap:RevolvingCreditFacilityMembermir:TwoThousandAndTwentyOneCreditAgreementMember2021-01-012021-12-310001809987mir:TwoThousandAndTwentyOneCreditAgreementMembermir:TermLoanFacilityMember2021-12-310001809987us-gaap:LondonInterbankOfferedRateLIBORMembersrt:MinimumMembermir:TwoThousandAndTwentyOneCreditAgreementMembermir:TermLoanFacilityMember2021-01-012021-12-310001809987us-gaap:LondonInterbankOfferedRateLIBORMembersrt:MaximumMembermir:TwoThousandAndTwentyOneCreditAgreementMembermir:TermLoanFacilityMember2021-01-012021-12-310001809987mir:AdjustedLiborMembermir:TwoThousandAndTwentyOneCreditAgreementMembermir:TermLoanFacilityMember2021-12-310001809987mir:TwoThousandAndTwentyOneCreditAgreementMembermir:TermLoanFacilityMember2021-01-012021-12-310001809987us-gaap:LondonInterbankOfferedRateLIBORMembersrt:MaximumMemberus-gaap:RevolvingCreditFacilityMembermir:TwoThousandAndTwentyOneCreditAgreementMember2021-01-012021-12-310001809987us-gaap:LetterOfCreditMembermir:TwoThousandAndTwentyOneCreditAgreementMember2021-12-310001809987mir:TwoThousandAndNineteenCreditFacilityMemberus-gaap:SecuredDebtMember2019-12-310001809987mir:TwoThousandAndNineteenCreditFacilityMembermir:TermLoanFacilityMember2019-12-310001809987us-gaap:RevolvingCreditFacilityMembermir:TwoThousandAndNineteenCreditFacilityMember2019-12-310001809987mir:UsdTermLoanMembermir:TwoThousandAndNineteenCreditFacilityMember2020-12-310001809987mir:UsdTermLoanMembermir:TwoThousandAndNineteenCreditFacilityMember2021-07-310001809987mir:UsdTermLoanMembermir:TwoThousandAndNineteenCreditFacilityMember2019-12-310001809987us-gaap:LondonInterbankOfferedRateLIBORMembersrt:MaximumMembermir:UsdTermLoanMembermir:TwoThousandAndNineteenCreditFacilityMember2019-01-012019-12-310001809987mir:UsdTermLoanMembermir:AdjustedLiborMembermir:TwoThousandAndNineteenCreditFacilityMember2021-10-200001809987mir:UsdTermLoanMembermir:AdjustedLiborMembermir:TwoThousandAndNineteenCreditFacilityMember2021-06-300001809987mir:UsdTermLoanMembermir:AdjustedLiborMembermir:TwoThousandAndNineteenCreditFacilityMember2020-06-300001809987mir:UsdTermLoanMembermir:AdjustedLiborMembermir:TwoThousandAndNineteenCreditFacilityMember2020-07-012021-06-300001809987mir:UsdTermLoanMembermir:AdjustedLiborMembermir:TwoThousandAndNineteenCreditFacilityMember2019-07-012020-06-300001809987mir:UsdTermLoanMembermir:AdjustedLiborMembermir:TwoThousandAndNineteenCreditFacilityMember2021-07-012021-10-310001809987us-gaap:EurodollarMembermir:TwoThousandAndNineteenCreditFacilityMembermir:EuroTermLoanMember2019-12-310001809987us-gaap:LondonInterbankOfferedRateLIBORMembersrt:MaximumMembermir:TwoThousandAndNineteenCreditFacilityMembermir:EuroTermLoanMember2019-01-012019-12-310001809987mir:TwoThousandAndNineteenCreditFacilityMembermir:EuroTermLoanMember2019-12-310001809987us-gaap:EurodollarMembermir:TwoThousandAndNineteenCreditFacilityMembermir:EuroTermLoanMember2020-06-300001809987us-gaap:EurodollarMembermir:TwoThousandAndNineteenCreditFacilityMembermir:EuroTermLoanMember2021-10-200001809987us-gaap:EurodollarMembermir:TwoThousandAndNineteenCreditFacilityMembermir:EuroTermLoanMember2021-06-300001809987us-gaap:EurodollarMembermir:TwoThousandAndNineteenCreditFacilityMembermir:EuroTermLoanMember2020-07-012021-06-300001809987us-gaap:EurodollarMembermir:TwoThousandAndNineteenCreditFacilityMembermir:EuroTermLoanMember2019-07-012020-06-300001809987us-gaap:EurodollarMembermir:TwoThousandAndNineteenCreditFacilityMembermir:EuroTermLoanMember2021-07-012021-10-190001809987us-gaap:LondonInterbankOfferedRateLIBORMembersrt:MaximumMemberus-gaap:RevolvingCreditFacilityMembermir:TwoThousandAndNineteenCreditFacilityMember2019-01-012019-12-310001809987us-gaap:RevolvingCreditFacilityMembermir:TwoThousandAndNineteenCreditFacilityMember2019-01-012019-12-310001809987us-gaap:LetterOfCreditMembermir:TwoThousandAndNineteenCreditFacilityMember2021-06-300001809987us-gaap:LetterOfCreditMembermir:TwoThousandAndNineteenCreditFacilityMember2020-06-300001809987us-gaap:RevolvingCreditFacilityMembermir:TwoThousandAndNineteenCreditFacilityMember2021-06-300001809987us-gaap:RevolvingCreditFacilityMembermir:TwoThousandAndNineteenCreditFacilityMember2020-06-300001809987mir:TwoThousandAndTwentyOneCreditAgreementMembermir:TermLoanFacilityMember2019-01-012019-12-310001809987us-gaap:RevolvingCreditFacilityMembermir:TwoThousandAndTwentyOneCreditAgreementMember2019-01-012019-12-310001809987mir:TwoThousandAndNineteenCreditFacilityMember2019-06-300001809987mir:CreditFacilityAmendmentAgreementMembermir:IncrementalProceedsTwoThousandAndTwentyOneMembermir:TwoThousandAndNineteenCreditFacilityMembermir:UsdAndEuroTermLoanMember2018-07-012019-06-300001809987mir:CreditFacilityAmendmentAgreementMembermir:TwoThousandAndNineteenCreditFacilityMembermir:UsdAndEuroTermLoanMembermir:IncrementalProceedsTwoThousandAndTwentyMember2018-07-012019-06-3000018099872019-03-012019-03-310001809987mir:TwoThousandAndNineteenCreditFacilityMembermir:PreviousRevolvingCreditAgreementMember2019-03-012019-03-3100018099872019-01-012019-12-310001809987mir:TwoThousandAndNineteenCreditFacilityMembermir:NrgLoanMember2021-06-300001809987mir:TwoThousandAndNineteenCreditFacilityMember2021-06-300001809987mir:CanadianFinancialInstitutionMembermir:TwoThousandAndNineteenCreditFacilityMember2019-05-31iso4217:CAD0001809987mir:TwoThousandAndNineteenCreditFacilityMembermir:JlgNotePayableMember2019-05-310001809987mir:TwoThousandAndNineteenCreditFacilityMembermir:JlgNotePayableMember2021-06-300001809987us-gaap:LetterOfCreditMember2021-12-310001809987us-gaap:LetterOfCreditMember2021-06-300001809987us-gaap:LetterOfCreditMember2020-06-300001809987us-gaap:LetterOfCreditMember2021-01-012021-12-310001809987us-gaap:LetterOfCreditMember2020-07-012021-06-300001809987us-gaap:LetterOfCreditMember2019-07-012020-06-300001809987us-gaap:LetterOfCreditMembersrt:MinimumMember2021-12-310001809987us-gaap:LetterOfCreditMembersrt:MaximumMember2021-12-310001809987mir:ShareholderMember2021-12-310001809987mir:ShareholderMember2021-06-300001809987mir:ShareholderMember2020-06-300001809987srt:ManagementMember2021-12-310001809987srt:ManagementMember2021-06-300001809987srt:ManagementMember2020-06-300001809987mir:ShareholderMembermir:PlusAccruedPaidInKindInterestNotesMember2019-01-012019-12-310001809987srt:ManagementMembermir:PlusAccruedPaidInKindInterestNotesMember2019-01-012019-12-310001809987mir:ShareholderMembermir:PlusAccruedPaidInKindInterestNotesMember2020-07-012021-06-300001809987mir:ShareholderMembermir:PlusAccruedPaidInKindInterestNotesMember2019-07-012020-06-300001809987mir:ShareholderMembermir:PlusAccruedPaidInKindInterestNotesMember2021-06-300001809987srt:ManagementMembermir:PlusAccruedPaidInKindInterestNotesMember2020-07-012021-06-300001809987srt:ManagementMembermir:PlusAccruedPaidInKindInterestNotesMember2019-07-012020-06-300001809987mir:ShareholderMembermir:PlusAccruedPaidInKindInterestNotesMember2021-10-0100018099872021-10-010001809987mir:PlusAccruedPaidInKindCashAndInterestNotesMembersrt:ManagementMember2020-07-012021-06-300001809987mir:PlusAccruedPaidInKindCashAndInterestNotesMembersrt:ManagementMember2019-07-012020-06-300001809987mir:ShareholderMembermir:PlusAccruedPaidInKindInterestNotesMember2020-01-012020-12-310001809987srt:ManagementMembermir:PlusAccruedPaidInKindInterestNotesMember2020-01-012020-12-310001809987srt:MinimumMember2021-12-310001809987srt:MaximumMember2021-12-3100018099872021-12-302021-12-300001809987country:US2021-10-202021-12-310001809987mir:ForeignMember2021-10-202021-12-310001809987country:GB2021-07-012021-10-190001809987country:GB2020-07-012021-06-300001809987country:GB2019-07-012020-06-300001809987country:GB2018-07-012019-06-300001809987country:US2021-07-012021-10-190001809987country:US2020-07-012021-06-300001809987country:US2019-07-012020-06-300001809987country:US2018-07-012019-06-300001809987mir:OtherForeignMember2021-07-012021-10-190001809987mir:OtherForeignMember2020-07-012021-06-300001809987mir:OtherForeignMember2019-07-012020-06-300001809987mir:OtherForeignMember2018-07-012019-06-300001809987mir:UsControlledForeignCorporationsMember2021-12-310001809987mir:NonUsControlledForeignCorporationsMember2021-12-310001809987us-gaap:DomesticCountryMember2021-12-310001809987us-gaap:StateAndLocalJurisdictionMember2021-12-310001809987us-gaap:ForeignCountryMember2021-12-310001809987mir:A2035Memberus-gaap:DomesticCountryMember2021-01-012021-12-310001809987us-gaap:DomesticCountryMembermir:A2036Member2021-01-012021-12-310001809987us-gaap:StateAndLocalJurisdictionMembermir:A2022Member2021-01-012021-12-310001809987us-gaap:StateAndLocalJurisdictionMembermir:A2041Member2021-01-012021-12-310001809987mir:A2023Member2021-01-012021-12-310001809987mir:A2037Member2021-01-012021-12-310001809987mir:NoncurrentIncomeTaxPayableMember2021-12-310001809987mir:NoncurrentIncomeTaxPayableMember2021-06-300001809987mir:NoncurrentIncomeTaxPayableMember2020-06-300001809987us-gaap:CanadaRevenueAgencyMemberus-gaap:EarliestTaxYearMemberus-gaap:ForeignCountryMember2021-01-012021-12-310001809987us-gaap:CanadaRevenueAgencyMemberus-gaap:LatestTaxYearMemberus-gaap:ForeignCountryMember2021-01-012021-12-310001809987us-gaap:MinistryOfTheEconomyFinanceAndIndustryFranceMemberus-gaap:EarliestTaxYearMemberus-gaap:ForeignCountryMember2021-01-012021-12-310001809987us-gaap:MinistryOfTheEconomyFinanceAndIndustryFranceMemberus-gaap:LatestTaxYearMemberus-gaap:ForeignCountryMember2021-01-012021-12-310001809987us-gaap:FederalMinistryOfFinanceGermanyMemberus-gaap:EarliestTaxYearMemberus-gaap:ForeignCountryMember2021-01-012021-12-310001809987us-gaap:FederalMinistryOfFinanceGermanyMemberus-gaap:LatestTaxYearMemberus-gaap:ForeignCountryMember2021-01-012021-12-310001809987us-gaap:HerMajestysRevenueAndCustomsHMRCMemberus-gaap:EarliestTaxYearMemberus-gaap:ForeignCountryMember2021-01-012021-12-310001809987us-gaap:HerMajestysRevenueAndCustomsHMRCMemberus-gaap:LatestTaxYearMemberus-gaap:ForeignCountryMember2021-01-012021-12-310001809987us-gaap:InternalRevenueServiceIRSMemberus-gaap:DomesticCountryMemberus-gaap:EarliestTaxYearMember2021-01-012021-12-310001809987us-gaap:InternalRevenueServiceIRSMemberus-gaap:DomesticCountryMemberus-gaap:LatestTaxYearMember2021-01-012021-12-310001809987us-gaap:StateAndLocalJurisdictionMemberus-gaap:InternalRevenueServiceIRSMemberus-gaap:EarliestTaxYearMember2021-01-012021-12-310001809987us-gaap:StateAndLocalJurisdictionMemberus-gaap:InternalRevenueServiceIRSMemberus-gaap:LatestTaxYearMember2021-01-012021-12-310001809987us-gaap:UnfundedPlanMemberus-gaap:PensionPlansDefinedBenefitMember2021-12-310001809987us-gaap:UnfundedPlanMemberus-gaap:PensionPlansDefinedBenefitMember2021-06-300001809987us-gaap:UnfundedPlanMemberus-gaap:PensionPlansDefinedBenefitMember2020-06-300001809987us-gaap:PensionPlansDefinedBenefitMember2021-10-202021-12-310001809987us-gaap:PensionPlansDefinedBenefitMember2021-07-012021-10-190001809987us-gaap:PensionPlansDefinedBenefitMember2020-07-012021-06-300001809987us-gaap:PensionPlansDefinedBenefitMember2019-07-012020-06-300001809987us-gaap:PensionPlansDefinedBenefitMember2018-07-012019-06-300001809987us-gaap:UnfundedPlanMemberus-gaap:OtherPostretirementBenefitPlansDefinedBenefitMember2021-12-310001809987us-gaap:UnfundedPlanMemberus-gaap:OtherPostretirementBenefitPlansDefinedBenefitMember2021-06-300001809987us-gaap:UnfundedPlanMemberus-gaap:OtherPostretirementBenefitPlansDefinedBenefitMember2020-06-300001809987mir:A2021OmnibusIncentivePlanMemberus-gaap:CommonClassAMember2021-10-190001809987mir:A2021OmnibusIncentivePlanMember2021-12-310001809987mir:A2021OmnibusIncentivePlanMember2021-01-012021-12-310001809987us-gaap:EmployeeStockMember2021-10-202021-12-310001809987us-gaap:EmployeeStockMember2021-07-012021-10-190001809987us-gaap:RestrictedStockMembersrt:MinimumMember2021-01-012021-12-310001809987srt:MaximumMemberus-gaap:RestrictedStockMember2021-01-012021-12-310001809987mir:PerformanceBasedRestrictedStockUnitsMembersrt:MinimumMember2021-10-190001809987srt:MaximumMembermir:PerformanceBasedRestrictedStockUnitsMember2021-10-190001809987mir:PerformanceBasedRestrictedStockUnitsMember2021-12-310001809987mir:PerformanceBasedRestrictedStockUnitsMember2021-01-012021-12-310001809987us-gaap:RestrictedStockUnitsRSUMember2021-10-190001809987mir:PerformanceBasedRestrictedStockUnitsMember2021-10-190001809987mir:DirectorRestrictedStockUnitsMember2021-10-190001809987us-gaap:RestrictedStockUnitsRSUMember2021-10-202021-12-310001809987mir:PerformanceBasedRestrictedStockUnitsMember2021-10-202021-12-310001809987mir:DirectorRestrictedStockUnitsMember2021-10-202021-12-310001809987us-gaap:RestrictedStockUnitsRSUMember2021-12-310001809987mir:DirectorRestrictedStockUnitsMember2021-12-310001809987mir:ThomasLoganCEOMember2021-06-172021-06-170001809987mir:BrianSchopferCFOMember2021-06-172021-06-170001809987mir:LawrenceKingsleyChairmanOfBoardMember2021-06-172021-06-1700018099872021-06-170001809987srt:MinimumMember2021-06-172021-06-170001809987srt:MaximumMember2021-06-172021-06-170001809987mir:ProfitInterest1Member2021-06-170001809987mir:ProfitInterest2Member2021-06-170001809987mir:ProfitInterest3Member2021-06-170001809987mir:ProfitsInterestsMember2021-01-012021-12-310001809987us-gaap:RestrictedStockMembermir:OrdinaryASharesOfMirionTopcoCommonStockMember2021-10-192021-10-190001809987us-gaap:RestrictedStockMember2021-10-192021-10-190001809987us-gaap:RestrictedStockMember2021-07-012021-10-190001809987us-gaap:RestrictedStockMember2020-07-012021-06-300001809987us-gaap:RestrictedStockMember2019-06-300001809987us-gaap:RestrictedStockMember2019-07-012020-06-300001809987us-gaap:RestrictedStockMember2020-06-300001809987us-gaap:RestrictedStockMember2021-06-300001809987us-gaap:RestrictedStockMember2021-10-190001809987us-gaap:RestrictedStockMember2018-07-012019-06-300001809987us-gaap:CommonClassBMember2020-08-130001809987us-gaap:CommonClassAMember2020-08-130001809987us-gaap:ShareBasedCompensationAwardTrancheOneMemberus-gaap:CommonClassAMember2021-10-202021-10-200001809987us-gaap:ShareBasedCompensationAwardTrancheTwoMemberus-gaap:CommonClassAMember2021-10-202021-10-200001809987us-gaap:ShareBasedCompensationAwardTrancheThreeMemberus-gaap:CommonClassAMember2021-10-202021-10-200001809987mir:GsDcSponsorILlcMemberus-gaap:PrivatePlacementMember2021-12-310001809987mir:GsDcSponsorILlcMemberus-gaap:PrivatePlacementMember2021-01-012021-12-3100018099872020-08-13utr:D00018099872020-08-132020-08-130001809987mir:SubscriptionAgreementMembermir:GoldmanSachsAssetManagementMemberus-gaap:CommonClassAMember2021-01-012021-12-3100018099872020-11-122020-11-120001809987mir:AffiliateOfTheSponsorMember2021-01-012021-12-310001809987srt:MaximumMember2021-06-300001809987mir:AffiliateOfTheSponsorMember2021-06-300001809987mir:CharterhouseCapitalPartnersLLPMember2021-01-012021-12-310001809987mir:CharterhouseCapitalPartnersLLPMember2018-07-012019-06-300001809987mir:CharterhouseCapitalPartnersLLPMember2019-07-012020-06-300001809987mir:CharterhouseCapitalPartnersLLPMember2020-07-012021-06-300001809987us-gaap:OperatingSegmentsMembermir:MedicalSegmentMember2021-10-202021-12-310001809987us-gaap:OperatingSegmentsMembermir:MedicalSegmentMember2021-07-012021-10-190001809987us-gaap:OperatingSegmentsMembermir:MedicalSegmentMember2020-07-012021-06-300001809987us-gaap:OperatingSegmentsMembermir:MedicalSegmentMember2019-07-012020-06-300001809987us-gaap:OperatingSegmentsMembermir:MedicalSegmentMember2018-07-012019-06-300001809987us-gaap:OperatingSegmentsMembermir:IndustrialSegmentMember2021-10-202021-12-310001809987us-gaap:OperatingSegmentsMembermir:IndustrialSegmentMember2021-07-012021-10-190001809987us-gaap:OperatingSegmentsMembermir:IndustrialSegmentMember2020-07-012021-06-300001809987us-gaap:OperatingSegmentsMembermir:IndustrialSegmentMember2019-07-012020-06-300001809987us-gaap:OperatingSegmentsMembermir:IndustrialSegmentMember2018-07-012019-06-300001809987us-gaap:OperatingSegmentsMember2021-10-202021-12-310001809987us-gaap:OperatingSegmentsMember2021-07-012021-10-190001809987us-gaap:OperatingSegmentsMember2020-07-012021-06-300001809987us-gaap:OperatingSegmentsMember2019-07-012020-06-300001809987us-gaap:OperatingSegmentsMember2018-07-012019-06-300001809987us-gaap:OperatingSegmentsMemberus-gaap:CorporateMember2021-10-202021-12-310001809987us-gaap:OperatingSegmentsMemberus-gaap:CorporateMember2021-07-012021-10-190001809987us-gaap:OperatingSegmentsMemberus-gaap:CorporateMember2020-07-012021-06-300001809987us-gaap:OperatingSegmentsMemberus-gaap:CorporateMember2019-07-012020-06-300001809987us-gaap:OperatingSegmentsMemberus-gaap:CorporateMember2018-07-012019-06-300001809987us-gaap:CorporateAndOtherMemberus-gaap:OperatingSegmentsMember2021-10-202021-12-310001809987us-gaap:CorporateAndOtherMemberus-gaap:OperatingSegmentsMember2021-07-012021-10-190001809987us-gaap:CorporateAndOtherMemberus-gaap:OperatingSegmentsMember2020-07-012021-06-300001809987us-gaap:CorporateAndOtherMemberus-gaap:OperatingSegmentsMember2019-07-012020-06-300001809987us-gaap:CorporateAndOtherMemberus-gaap:OperatingSegmentsMember2018-07-012019-06-300001809987srt:NorthAmericaMembermir:MedicalSegmentMember2021-10-202021-12-310001809987srt:NorthAmericaMembermir:MedicalSegmentMember2021-07-012021-10-190001809987srt:NorthAmericaMembermir:MedicalSegmentMember2020-07-012021-06-300001809987srt:NorthAmericaMembermir:MedicalSegmentMember2019-07-012020-06-300001809987srt:NorthAmericaMembermir:MedicalSegmentMember2018-07-012019-06-300001809987srt:NorthAmericaMembermir:IndustrialSegmentMember2021-10-202021-12-310001809987srt:NorthAmericaMembermir:IndustrialSegmentMember2021-07-012021-10-190001809987srt:NorthAmericaMembermir:IndustrialSegmentMember2020-07-012021-06-300001809987srt:NorthAmericaMembermir:IndustrialSegmentMember2019-07-012020-06-300001809987srt:NorthAmericaMembermir:IndustrialSegmentMember2018-07-012019-06-300001809987srt:NorthAmericaMember2021-10-202021-12-310001809987srt:NorthAmericaMember2021-07-012021-10-190001809987srt:NorthAmericaMember2020-07-012021-06-300001809987srt:NorthAmericaMember2019-07-012020-06-300001809987srt:NorthAmericaMember2018-07-012019-06-300001809987srt:EuropeMembermir:MedicalSegmentMember2021-10-202021-12-310001809987srt:EuropeMembermir:MedicalSegmentMember2021-07-012021-10-190001809987srt:EuropeMembermir:MedicalSegmentMember2020-07-012021-06-300001809987srt:EuropeMembermir:MedicalSegmentMember2019-07-012020-06-300001809987srt:EuropeMembermir:MedicalSegmentMember2018-07-012019-06-300001809987srt:EuropeMembermir:IndustrialSegmentMember2021-10-202021-12-310001809987srt:EuropeMembermir:IndustrialSegmentMember2021-07-012021-10-190001809987srt:EuropeMembermir:IndustrialSegmentMember2020-07-012021-06-300001809987srt:EuropeMembermir:IndustrialSegmentMember2019-07-012020-06-300001809987srt:EuropeMembermir:IndustrialSegmentMember2018-07-012019-06-300001809987srt:EuropeMember2021-10-202021-12-310001809987srt:EuropeMember2021-07-012021-10-190001809987srt:EuropeMember2020-07-012021-06-300001809987srt:EuropeMember2019-07-012020-06-300001809987srt:EuropeMember2018-07-012019-06-300001809987srt:AsiaPacificMembermir:MedicalSegmentMember2021-10-202021-12-310001809987srt:AsiaPacificMembermir:MedicalSegmentMember2021-07-012021-10-190001809987srt:AsiaPacificMembermir:MedicalSegmentMember2020-07-012021-06-300001809987srt:AsiaPacificMembermir:MedicalSegmentMember2019-07-012020-06-300001809987srt:AsiaPacificMembermir:MedicalSegmentMember2018-07-012019-06-300001809987mir:IndustrialSegmentMembersrt:AsiaPacificMember2021-10-202021-12-310001809987mir:IndustrialSegmentMembersrt:AsiaPacificMember2021-07-012021-10-190001809987mir:IndustrialSegmentMembersrt:AsiaPacificMember2020-07-012021-06-300001809987mir:IndustrialSegmentMembersrt:AsiaPacificMember2019-07-012020-06-300001809987mir:IndustrialSegmentMembersrt:AsiaPacificMember2018-07-012019-06-300001809987srt:AsiaPacificMember2021-10-202021-12-310001809987srt:AsiaPacificMember2021-07-012021-10-190001809987srt:AsiaPacificMember2020-07-012021-06-300001809987srt:AsiaPacificMember2019-07-012020-06-300001809987srt:AsiaPacificMember2018-07-012019-06-300001809987country:FR2021-10-202021-12-310001809987country:FR2021-07-012021-10-190001809987country:FR2020-07-012021-06-300001809987country:FR2019-07-012020-06-300001809987country:FR2018-07-012019-06-300001809987us-gaap:TransferredAtPointInTimeMember2021-10-202021-12-310001809987us-gaap:TransferredAtPointInTimeMember2021-07-012021-10-190001809987us-gaap:TransferredAtPointInTimeMember2020-07-012021-06-300001809987us-gaap:TransferredAtPointInTimeMember2019-07-012020-06-300001809987us-gaap:TransferredAtPointInTimeMember2018-07-012019-06-300001809987us-gaap:TransferredOverTimeMember2021-10-202021-12-310001809987us-gaap:TransferredOverTimeMember2021-07-012021-10-190001809987us-gaap:TransferredOverTimeMember2020-07-012021-06-300001809987us-gaap:TransferredOverTimeMember2019-07-012020-06-300001809987us-gaap:TransferredOverTimeMember2018-07-012019-06-300001809987mir:MedicalMembermir:MedicalSegmentMember2021-10-202021-12-310001809987mir:MedicalMembermir:MedicalSegmentMember2021-07-012021-10-190001809987mir:MedicalMembermir:MedicalSegmentMember2020-07-012021-06-300001809987mir:MedicalMembermir:MedicalSegmentMember2019-07-012020-06-300001809987mir:MedicalMembermir:MedicalSegmentMember2018-07-012019-06-300001809987mir:ReactorSafetyAndControlSystemsMembermir:IndustrialSegmentMember2021-10-202021-12-310001809987mir:ReactorSafetyAndControlSystemsMembermir:IndustrialSegmentMember2021-07-012021-10-190001809987mir:ReactorSafetyAndControlSystemsMembermir:IndustrialSegmentMember2020-07-012021-06-300001809987mir:ReactorSafetyAndControlSystemsMembermir:IndustrialSegmentMember2019-07-012020-06-300001809987mir:ReactorSafetyAndControlSystemsMembermir:IndustrialSegmentMember2018-07-012019-06-300001809987mir:RadiologicalSearchMeasurementAndAnalysisSystemsMembermir:IndustrialSegmentMember2021-10-202021-12-310001809987mir:RadiologicalSearchMeasurementAndAnalysisSystemsMembermir:IndustrialSegmentMember2021-07-012021-10-190001809987mir:RadiologicalSearchMeasurementAndAnalysisSystemsMembermir:IndustrialSegmentMember2020-07-012021-06-300001809987mir:RadiologicalSearchMeasurementAndAnalysisSystemsMembermir:IndustrialSegmentMember2019-07-012020-06-300001809987mir:RadiologicalSearchMeasurementAndAnalysisSystemsMembermir:IndustrialSegmentMember2018-07-012019-06-300001809987srt:NorthAmericaMember2021-12-310001809987srt:NorthAmericaMember2021-06-300001809987srt:NorthAmericaMember2020-06-300001809987srt:EuropeMember2021-12-310001809987srt:EuropeMember2021-06-300001809987srt:EuropeMember2020-06-300001809987srt:AsiaPacificMember2021-12-310001809987srt:AsiaPacificMember2021-06-300001809987srt:AsiaPacificMember2020-06-300001809987us-gaap:FairValueInputsLevel1Memberus-gaap:FairValueMeasurementsRecurringMembermir:CashCashEquivalentsAndRestrictedCashMember2021-12-310001809987us-gaap:FairValueMeasurementsRecurringMemberus-gaap:FairValueInputsLevel2Membermir:CashCashEquivalentsAndRestrictedCashMember2021-12-310001809987us-gaap:FairValueMeasurementsRecurringMemberus-gaap:FairValueInputsLevel3Membermir:CashCashEquivalentsAndRestrictedCashMember2021-12-310001809987mir:DiscretionaryRetirementPlanMemberus-gaap:FairValueInputsLevel1Memberus-gaap:FairValueMeasurementsRecurringMember2021-12-310001809987mir:DiscretionaryRetirementPlanMemberus-gaap:FairValueMeasurementsRecurringMemberus-gaap:FairValueInputsLevel2Member2021-12-310001809987mir:DiscretionaryRetirementPlanMemberus-gaap:FairValueMeasurementsRecurringMemberus-gaap:FairValueInputsLevel3Member2021-12-310001809987us-gaap:FairValueInputsLevel1Memberus-gaap:FairValueMeasurementsRecurringMember2021-12-310001809987us-gaap:FairValueMeasurementsRecurringMemberus-gaap:FairValueInputsLevel2Member2021-12-310001809987us-gaap:FairValueMeasurementsRecurringMemberus-gaap:FairValueInputsLevel3Member2021-12-310001809987us-gaap:FairValueInputsLevel1Memberus-gaap:FairValueMeasurementsRecurringMembermir:CashCashEquivalentsAndRestrictedCashMember2021-06-300001809987us-gaap:FairValueMeasurementsRecurringMemberus-gaap:FairValueInputsLevel2Membermir:CashCashEquivalentsAndRestrictedCashMember2021-06-300001809987us-gaap:FairValueMeasurementsRecurringMemberus-gaap:FairValueInputsLevel3Membermir:CashCashEquivalentsAndRestrictedCashMember2021-06-300001809987mir:DiscretionaryRetirementPlanMemberus-gaap:FairValueInputsLevel1Memberus-gaap:FairValueMeasurementsRecurringMember2021-06-300001809987mir:DiscretionaryRetirementPlanMemberus-gaap:FairValueMeasurementsRecurringMemberus-gaap:FairValueInputsLevel2Member2021-06-300001809987mir:DiscretionaryRetirementPlanMemberus-gaap:FairValueMeasurementsRecurringMemberus-gaap:FairValueInputsLevel3Member2021-06-300001809987us-gaap:FairValueInputsLevel1Memberus-gaap:FairValueMeasurementsRecurringMembermir:CashCashEquivalentsAndRestrictedCashMember2020-06-300001809987us-gaap:FairValueMeasurementsRecurringMemberus-gaap:FairValueInputsLevel2Membermir:CashCashEquivalentsAndRestrictedCashMember2020-06-300001809987us-gaap:FairValueMeasurementsRecurringMemberus-gaap:FairValueInputsLevel3Membermir:CashCashEquivalentsAndRestrictedCashMember2020-06-300001809987mir:DiscretionaryRetirementPlanMemberus-gaap:FairValueInputsLevel1Memberus-gaap:FairValueMeasurementsRecurringMember2020-06-300001809987mir:DiscretionaryRetirementPlanMemberus-gaap:FairValueMeasurementsRecurringMemberus-gaap:FairValueInputsLevel2Member2020-06-300001809987mir:DiscretionaryRetirementPlanMemberus-gaap:FairValueMeasurementsRecurringMemberus-gaap:FairValueInputsLevel3Member2020-06-300001809987us-gaap:FairValueInputsLevel1Memberus-gaap:FairValueMeasurementsRecurringMember2021-06-300001809987us-gaap:FairValueInputsLevel1Memberus-gaap:FairValueMeasurementsRecurringMember2020-06-300001809987us-gaap:EmployeeStockOptionMember2021-10-202021-12-310001809987us-gaap:EmployeeStockOptionMember2021-07-012021-10-190001809987us-gaap:EmployeeStockOptionMember2020-07-012021-06-300001809987us-gaap:EmployeeStockOptionMember2019-07-012020-06-300001809987us-gaap:EmployeeStockOptionMember2018-07-012019-06-300001809987us-gaap:SellingGeneralAndAdministrativeExpensesMember2021-10-202021-12-310001809987us-gaap:SellingGeneralAndAdministrativeExpensesMember2021-07-012021-10-190001809987us-gaap:SellingGeneralAndAdministrativeExpensesMember2020-07-012021-06-300001809987mir:IntermediatecoClassBCommonStockMember2021-10-202021-10-200001809987mir:IntermediatecoClassACommonStockMembermir:GsahMember2021-10-200001809987mir:IntermediatecoClassBCommonStockMember2021-10-200001809987srt:ParentCompanyMembermir:MirionTechnologiesLtdMember2021-12-310001809987srt:ParentCompanyMembermir:MirionTechnologiesLtdMember2021-10-202021-12-310001809987mir:MirionTechnologiesLtdMember2021-12-310001809987mir:MirionTechnologiesLtdMember2021-10-190001809987mir:MirionTechnologiesLtdMember2021-10-202021-10-200001809987srt:ParentCompanyMembermir:MirionTechnologiesLtdMember2021-06-300001809987srt:ParentCompanyMembermir:MirionTechnologiesLtdMember2020-06-300001809987srt:ParentCompanyMembermir:MirionTechnologiesLtdMember2021-07-012021-10-190001809987srt:ParentCompanyMembermir:MirionTechnologiesLtdMember2020-07-012021-06-300001809987srt:ParentCompanyMembermir:MirionTechnologiesLtdMember2019-07-012020-06-300001809987srt:ParentCompanyMembermir:MirionTechnologiesLtdMember2018-07-012019-06-300001809987mir:MirionTechnologiesLtdMember2020-06-300001809987mir:MirionTechnologiesLtdMember2021-06-300001809987mir:MirionTechnologiesLtdMember2019-06-300001809987us-gaap:AllowanceForCreditLossMember2020-12-310001809987us-gaap:AllowanceForCreditLossMember2021-01-012021-12-310001809987us-gaap:AllowanceForCreditLossMember2021-12-310001809987us-gaap:WarrantyReservesMember2020-12-310001809987us-gaap:WarrantyReservesMember2021-01-012021-12-310001809987us-gaap:WarrantyReservesMember2021-12-310001809987us-gaap:AllowanceForCreditLossMember2021-06-300001809987us-gaap:AllowanceForCreditLossMember2021-07-012021-10-190001809987us-gaap:AllowanceForCreditLossMember2021-10-190001809987us-gaap:WarrantyReservesMember2021-06-300001809987us-gaap:WarrantyReservesMember2021-07-012021-10-190001809987us-gaap:WarrantyReservesMember2021-10-190001809987us-gaap:AllowanceForCreditLossMember2020-06-300001809987us-gaap:AllowanceForCreditLossMember2020-07-012021-06-300001809987us-gaap:WarrantyReservesMember2020-06-300001809987us-gaap:WarrantyReservesMember2020-07-012021-06-300001809987us-gaap:AllowanceForCreditLossMember2019-06-300001809987us-gaap:AllowanceForCreditLossMember2019-07-012020-06-300001809987us-gaap:WarrantyReservesMember2019-06-300001809987us-gaap:WarrantyReservesMember2019-07-012020-06-300001809987us-gaap:AllowanceForCreditLossMember2018-06-300001809987us-gaap:AllowanceForCreditLossMember2018-07-012019-06-300001809987us-gaap:WarrantyReservesMember2018-06-300001809987us-gaap:WarrantyReservesMember2018-07-012019-06-30
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 10-K
☒ ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
For the fiscal year ended December 31, 2021
OR
☐ TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
For the transition period from __________ to __________
Commission File Number: 001-39352
Mirion Technologies, Inc.
(Exact name of registrant as specified in its charter)
| | | | | | | | |
| Delaware | | 83-0974996 |
(State or other jurisdiction of incorporation or organization) |
| (I.R.S. Employer Identification Number) |
1218 Menlo Drive
Atlanta, Georgia 30318
(Address of Principal Executive Office)
(770) 432-2744
(Registrant's telephone number, including area code)
Securities registered pursuant to Section 12(b) of the Act: | | | | | | | | |
Title of each class | Trading symbol(s) | Name of each exchange on which registered |
Class A Common Stock, $0.0001 par value per share | MIR | New York Stock Exchange |
Redeemable warrants, each exercisable for one share of Class A common stock at an exercise price of $11.50 | MIR WS | New York Stock Exchange |
Securities registered pursuant to Section 12(g) of the Act: None
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. ☐ Yes ☒ No
Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Act. ☐ Yes ☒ No
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. ☒ Yes ☐ No
Indicate by check mark whether the registrant has submitted electronically every Interactive Data File required to be submitted pursuant to Rule 405 of Regulation S-T (§232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit such files). ☒ Yes ☐ No
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, a smaller reporting company, or an emerging growth company. See the definitions of “large accelerated filer,” “accelerated filer,” “smaller reporting company,” and “emerging growth company” in Rule 12b-2 of the Exchange Act.
| | | | | | | | | | | |
| Large Accelerated Filer | ☒ | Accelerated Filer | ☐ |
| | | |
| Non-accelerated Filer | ☐ | Smaller Reporting Company | ☐ |
| | | |
| | | Emerging Growth Company | ☐ |
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act). o
Indicate by check mark whether the registrant has filed a report on and attestation to its management’s assessment of the effectiveness of its internal control over financial reporting under Section 404(b) of the Sarbanes-Oxley Act (15 U.S.C. 7262(b)) by the registered public accounting firm that prepared or issued its audit report. x
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Exchange Act). o Yes x No
The aggregate market value of voting and non-voting common stock held by non-affiliates of the registrant (for this purpose, executive officers and directors of the registrant are considered affiliates) as of June 30, 2021 (the last business day of the most recently completed second quarter) was approximately $780,000,000.
Number of shares of the registrant’s Class A common stock outstanding at February 22, 2022: 199,523,292.
Number of shares of the registrant’s Class B common stock outstanding at February 22, 2022: 8,560,540.
CAUTIONARY NOTE REGARDING FORWARD-LOOKING STATEMENTS
This Annual Report on Form 10-K contains forward-looking statements within the meaning of the "safe-harbor" provisions of the Private Securities Litigation Reform Act of 1995 that reflect future plans, estimates, beliefs, and expected performance. All statements contained in this Annual Report on Form 10-K other than statements of historical fact, including statements regarding our future operating results and financial position, our business strategy and plans and our objectives for future operations, are forward-looking statements. This includes, without limitation, statements under “Part II, Item 7. Management’s Discussion and Analysis of Financial Condition and Results of Operations” regarding our financial position, capital structure, indebtedness, business strategy and the plans and objectives of management for future operations, market share and products sales, future market opportunities, future manufacturing capabilities and facilities, future sales channels and strategies. These statements constitute projections, forecasts and forward-looking statements, and are not guarantees of performance. When used in this Annual Report on Form 10-K, words such as “anticipate,” “believe,” “continue,” “could,” “estimate,” “expect,” “intend,” “may,” “might,” “plan,” “possible,” “potential,” “predict,” “project,” “should,” “strive,” “seeks,” “plans,” “scheduled,” “would” and similar expressions may identify forward-looking statements, but the absence of these words does not mean that a statement is not forward-looking. When we discuss our strategies or plans we are making projections, forecasts or forward-looking statements. Such statements are based on the beliefs of, as well as assumptions made by and information currently available to, our management.
The forward-looking statements contained in this Annual Report on Form 10-K are based on our current expectations and beliefs concerning future developments and their potential effects on us. There can be no assurance that future developments affecting us will be those that we have anticipated. These forward-looking statements involve a number of risks, uncertainties (some of which are beyond our control) or other assumptions that may cause actual results or performance to be materially different from those expressed or implied by these forward-looking statements. These risks and uncertainties include, but are not limited to, the following risks, uncertainties and other factors:
•changes in domestic and foreign business, market, economic, financial, political and legal conditions;
•risks related to the continued growth of our end markets;
•our ability to win new customers and retain existing customers;
•our ability to realize sales expected from our backlog of orders and contracts;
•risks related to governmental contracts;
•our ability to mitigate risks associated with long-term fixed price contracts, including risks related to inflation;
•risks related to information technology disruption or security;
•risks related to the implementation and enhancement of information systems;
•our ability to manage our supply chain or difficulties with third-party manufacturers;
•risks related to competition;
•our ability to manage disruptions of, or changes in, our independent sales representatives, distributors and original equipment manufacturers;
•our ability to realize the expected benefit from any synergies from acquisitions or internal restructuring and improvement efforts;
•our ability to issue equity or equity-linked securities in the future;
•risks related to changes in tax law and ongoing tax audits;
•risks related to future legislation and regulation both in the United States and abroad;
•risks related to the costs or liabilities associated with product liability claims;
•our ability to attract, train and retain key members of its leadership team and other qualified personnel;
•risks related to the adequacy of our insurance coverage;
•our ability to benefit from future acquisitions; including our ability to realize the value of goodwill and intangible assets;
•risks related to the global scope of our operations, including operations in international and emerging markets;
•risks related to our exposure to fluctuations in foreign currency exchange rates;
•our ability to comply with various laws and regulations and the costs associated with legal compliance;
•risks related to the outcome of any litigation, government and regulatory proceedings, investigations and inquiries;
•risks related to our ability to protect or enforce our proprietary rights on which our business depends or third-party intellectual property infringement claims;
•liabilities associated with environmental, health and safety matters;
•our ability to predict our future operational results;
•risks associated with our limited history of operating as an independent company;
•the impact of the global COVID-19 pandemic, including the availability, acceptance and efficacy of vaccinations and laws and regulations with respect to vaccinations, on our projected results of operations, financial performance or other financial metrics, or on any of the foregoing risks; and
•other risks and uncertainties indicated in this Annual Report on Form 10-K, including those under the heading “Risk Factors,” and other documents filed or to be filed with the SEC by us.
There can be no assurance that future developments affecting us will be those that we have anticipated. These forward-looking statements involve a number of risks, uncertainties (some of which are beyond our control) or other assumptions that may cause actual results or performance to be materially different from those expressed or implied by these forward-looking statements. Should one or more of these risks or uncertainties materialize, or should any of our assumptions prove incorrect, actual results may vary in material respects from those projected in these forward-looking statements.
Forward-looking statements included in this Annual Report on Form 10-K speak only as of the date of this Annual Report on Form 10-K or any earlier date specified for such statements. We undertake no obligation to update or revise any forward-looking statements, whether as a result of new information, future events or otherwise, except as may be required under applicable securities laws.
We intend to announce material information to the public through the Mirion Investor Relations website, available at ir.mirion.com, SEC filings, press releases, public conference calls and public webcasts. We use these channels, as well as social media, to communicate with our investors, customers and the public about our company, our offerings and other issues. It is possible that the information we post on our website or social media could be deemed to be material information. As such, we encourage investors, the media, and others to follow the channels listed above, including the social media channels listed on our investor relations website, and to review the information disclosed through such channels. Any updates to the list of disclosure channels through which we will announce information will be posted on the investor relations website.
TABLE OF CONTENTS
CERTAIN DEFINED TERMS
Unless the context otherwise requires, all references in this Annual Report on Form 10-K to “Mirion,” the “Company,” “we,” “us” or “our” refer to Mirion Technologies, Inc. following the Business Combination, other than certain historical information which refers to the business of Mirion Technologies (TopCo), Ltd. (“Mirion TopCo”) prior to the consummation of the business combination (the “Business Combination”) of GS Acquisition Holdings Corp II (“GSAH”) with Mirion TopCo on October 20, 2021, pursuant to that certain Business Combination Agreement, dated June 17, 2021 (as amended, the “Business Combination Agreement”), by and among GSAH, Mirion and the other parties thereto. See “Part I, Item 1. Business—Business Combination Overview” for more information. In addition, as a result of the Business Combination, our financial statement presentation distinguishes Mirion TopCo as the “Predecessor” for periods prior to the closing of the Business Combination. Mirion, which includes consolidation of Mirion’s subsidiaries, is the “Successor” for periods after the closing of the Business Combination.
Unless otherwise stated in this Annual Report on Form 10-K or the context otherwise requires, references to:
“ASC” are to the Accounting Standards Codification;
“Board” and “Board of Directors” are to the board of directors of Mirion Technologies, Inc. following the closing of the Business Combination;
“Bylaws” are to the bylaws of Mirion Technologies, Inc. in effect as of the date of this Annual Report on Form 10-K;
“Charter” are to the certificate of incorporation of Mirion Technologies, Inc. in effect as of the date of this Annual Report on Form 10-K;
“Class A common stock” are to shares of Mirion’s common stock, par value $0.0001 per share;
“Class B common stock” are to shares of Mirion’s common stock, par value $0.0001 per share;
“common stock” are to the Class A common stock and Class B common stock;
“COVID-19” are to SARS-CoV-2 or COVID-19, and any evolutions thereof or any other epidemics, pandemics or disease outbreaks;
“DGCL” are to the General Corporation Law of the State of Delaware;
“Exchange Act” are to the Securities Exchange Act of 1934, as amended;
“fiscal 2021” are to the twelve months ended June 30, 2021;
“fiscal 2020” are to the twelve months ended June 30, 2020;
“fiscal 2019” are to the twelve months ended June 30, 2019;
“founder shares” are to the Founder Shares (as defined under Note 15, Related Party Transactions, in the notes to the financial statements included in this Annual Report on Form 10-K);
“GSAH” are to GS Acquisition Holdings Corp II, prior to the consummation of the Business Combination;
“IntermediateCo” are to Mirion IntermediateCo, Inc., a Delaware corporation direct subsidiary of Mirion;
“IntermediateCo Class A common stock” are to the shares of Class A common stock of IntermediateCo, par value $0.0001 per share;
“IntermediateCo Class B common stock” are to the shares of Class B common stock of IntermediateCo, par value $0.0001 per share;
“Mirion TopCo” are to Mirion Technologies (TopCo), Ltd;
"Predecessor Period" refers to all reported financial periods prior to the Business Combination Closing Date on October 20, 2021;
“Predecessor Stub Period” means the transition period preceding the Business Combination from July 1, 2021 through October 19, 2021;
“private placement warrants” are to the Private Placement Warrants (as defined under Note 15, Related Party Transactions in the notes to the financial statements included in this Annual Report on Form 10-K);
“public warrants” are to the Public Warrants (as defined under Note 15, Related Party Transactions, in the notes to the financial statements included in this Annual Report on Form 10-K);
“Sarbanes-Oxley Act” are to the Sarbanes-Oxley Act of 2002;
“Securities Act” are to the Securities Act of 1933, as amended;
“Sponsor” are to GS Sponsor II LLC, a Delaware limited liability company;
“Sponsor Agreement” are to the Second Amended and Restated Sponsor Agreement, dated as of October 20, 2021, by and among us, the Sponsor and the other parties thereto;
"Successor Period" refers to the period from the Closing Date, October 20, 2021, and ended on December 31, 2021; and
“warrants” are to the public warrants and private placement warrants.
PART I
ITEM 1. BUSINESS
Business Overview
Mirion provides products, services and software that allow our customers to safely leverage the power of ionizing radiation for the greater good of humanity. Our solutions have critical applications in the medical, nuclear energy and defense markets, as well as in laboratories and scientific research, analysis and space exploration. Many of our markets are characterized by the need to meet rigorous regulatory standards, design qualifications and operating requirements. Throughout our history, we have successfully leveraged the strength of our expertise in ionizing radiation to continually drive innovation and expand the commercial applications of our core technology competencies. Through our facilities in 13 countries, we supply our solutions in the Americas, Europe, Africa, the Middle East and Asia Pacific regions.
We are headquartered in Atlanta, Georgia and have operations in the United States, Canada, the United Kingdom, France, Germany, Finland, China, Belgium, Netherlands, Estonia, Japan, and South Korea.
We have two reportable business segments: Medical and Industrial. Our Medical segment supports applications in medical diagnostics, cancer treatment, practitioner safety and rehabilitation. Our products in these fields are focused on enhancing the effectiveness and safety of life-saving procedures. Our Industrial segment is focused on addressing critical radiation safety, measurement and analysis applications across nuclear energy, defense, laboratories and research and other industrial markets. Products and solutions of our Medical segment and of our Industrial segment include: dosimetry services (environmental radiation monitoring dose of records services), cancer diagnostics and therapy quality assurance, or "QA", nuclear medicine, dosimeters (wearable devices that measure exposure to ionizing radiation), contamination and clearance monitors, detection and identification instruments, radiation monitoring systems, electrical penetrations, reactor instrumentation and control equipment and systems, medical and industrial imaging systems and related accessories, software and services, alpha spectroscopy instruments (instruments that quantify and identify alpha-emitting nuclides), alpha/beta counting instruments (instruments for quantification of alpha and beta radiation) and gamma spectroscopy detector systems (instruments for qualification and quantification of gamma emitting nuclides) and software (related software to support our product and solution offerings).
For more than 60 years, we and our predecessor companies have delivered products and services that enable our customer to harness ionizing radiation for applications that benefit the health, safety, vitality and technological progress of humanity. We believe the breadth and proven performance of our solutions support our longstanding strategic customer partnerships across diverse end markets. Our products, software and services have been sold directly and indirectly to a variety of end-use customers, including, medical service providers, the vast majority of the U.S. nuclear power producers and the addressable global installed base of active nuclear power reactors, many of the leading nuclear reactor design firms, universities, numerous international government and supranational agencies, 19 of the 28 NATO militaries, national laboratories, environmental laboratories, research institutes and industrial companies.
Our broad product and services portfolio of medical, search, measurement, scientific analysis and reactor safety and control systems are supported by our engineering and research and development organization of 356 scientists, engineers and technicians, who represented approximately 14% of our workforce as of December 31, 2021. We possess numerous product qualifications, trade secrets and patents that support our market position and our ability to deliver next generation products and services. In addition, we maintain design, manufacturing and sales capabilities across 12 countries in America, Europe and Asia, enabling us to capitalize on growth opportunities, including the ongoing growth in spending for medical, defense and homeland security and the ongoing growth for nuclear power.
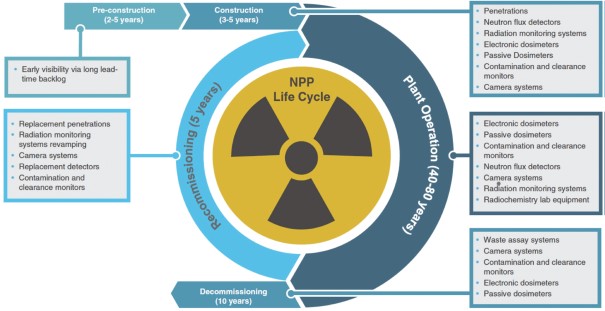
Our financial performance is driven by the replacement of products and the recurring provision of services into our core end markets, as well as the construction of new facilities like nuclear power plants, or NPPs, globally.
Business Combination Overview
On October 20, 2021 (the “Closing Date”), Mirion Technologies, Inc. (formerly known as GS Acquisition Holdings Corp II) consummated its previously announced Business Combination pursuant to the Business Combination Agreement. In connection with the Business Combination, stockholders of GSAH elected to redeem 14,628,610 shares of GSAH's Class A common stock, representing approximately 19.5% of GSAH's issued and outstanding Class A common stock before giving effect to the Business Combination.
In order to implement a structure similar to that of an “Up-C,” the Company formed IntermediateCo, and a newly-formed subsidiary of IntermediateCo merged with and into Mirion TopCo with Mirion TopCo surviving as a wholly-owned
subsidiary of IntermediateCo. The Company holds 100% of the shares of IntermediateCo Class A common stock, and greater than 80% of the shares of IntermediateCo Class B common stock. The shares of IntermediateCo Class B common stock not held by the Company are held by certain pre-Business Combination stockholders of Mirion TopCo, as described below.
The aggregate business combination consideration (the “Business Combination Consideration”) paid by the Company to the pre-Business Combination stockholders of Mirion TopCo (collectively, the "Mirion Sellers") in connection with the consummation of the Business Combination was approximately $1.3 billion in cash, 30,401,902 newly issued shares of Class A common stock and 8,560,540 newly issued shares of the Class B common stock. The Mirion Sellers receiving shares of Class B common stock also received one share of IntermediateCo Class B common stock per share of Class B common stock as a paired interest (the “paired interests”). Each share of Class A common stock and each paired interest were valued at $10.00 per share for purposes of determining the aggregate number of shares issued to the Mirion Sellers.
The holders of the founder shares agreed to waive the anti-dilution adjustments provided for in GSAH’s Amended and Restated Certificate of Incorporation, which were applicable to the Class B common stock. As a result of such waiver, the 18,750,000 founder shares automatically converted into shares of Class A common stock on a one-for-one basis upon the consummation of the Business Combination. Pursuant to the Sponsor Agreement, the founder shares also became subject to vesting in three equal tranches, based on the volume-weighted average price of the Class A common stock being greater than or equal to $12.00, $14.00 and $16.00 (each, a “Founder Share Vesting Event”) per share for any 20 trading days in any 30 consecutive trading day period. Vesting of the founder shares will be accelerated upon certain sale events based on the per share price of the Class A common stock in such sale event. Holders of the founder shares are entitled to vote such founder shares and receive dividends and other distributions with respect to such founder shares prior to vesting, but such dividends and other distributions with respect to unvested founder shares will be set aside and shall only be paid to the holders of the founder shares upon the vesting of such founder shares. The founder shares will be forfeited to us for no consideration if they fail to vest within five years of the Closing Date.
Concurrently with the execution of the Business Combination Agreement, GSAH entered into subscription agreements (the “Subscription Agreements”) with certain investors (collectively, the “PIPE Investors”), pursuant to, and on the terms and subject to the conditions of which, the PIPE Investors collectively subscribed for 90,000,000 shares of Class A common stock for an aggregate purchase price equal to $900,000,000 (the “PIPE Investment” and, such shares, the “PIPE Shares”). The PIPE Investment was consummated substantially concurrently with the Closing.
A subsidiary of Mirion TopCo, Mirion Technologies (HoldingSub1), Ltd. (“UKTopCo”), previously issued certain PIK Notes to certain Mirion TopCo stockholders and members of Mirion management (collectively, the “PIK Notes”). Substantially concurrent with the Closing, a portion of the Business Combination Consideration was used to extinguish the PIK Notes in full.
On October 20, 2021, the Board of Directors determined to change Mirion TopCo's fiscal year end from June 30 of each year to December 31 of each year in order to align Mirion’s fiscal year end with GSAH’s fiscal year end.
Industry Overview
We have two reportable business segments: Medical and Industrial. Our Medical segment is based around our sales, products and services to customers in the medical market. The Industrial segment is primarily based around the nuclear energy, defense, laboratories and scientific research markets as well as other industrial markets.
Medical
Our medical market is comprised of rapidly growing product applications in cancer diagnostics and therapeutics, nuclear medicine, dosimetry services and rehabilitation. We offer products, software and services in each of these areas that enhance the effectiveness and safety of life-saving procedures in these areas. According to the World Nuclear Association, or WNA, as of October 2021, there are over 10,000 hospitals worldwide using radioisotopes in medicine, with about 90% of the procedures for diagnostics, and more than 40 million procedures are performed globally every year, 20 million being in the United States and 10 million in Europe. The WNA also estimates that, as of April 2021, the use of radioactive substances, or radiopharmaceuticals, in diagnosis is growing at over 10% per year. In the radiotherapy market, demand is driven by replacement of the underlying linear accelerator, or Linac, installed base. As of 2019 there were approximately 14,000 Linacs deployed worldwide, and it is estimated that this will grow to approximately 16,500 Linacs worldwide by 2024, according to a global consulting firm.
Nuclear medicine is a medical specialty that uses radiopharmaceuticals to diagnose, monitor and treat disease. Our products address the complicated lifecycle of radiopharmaceuticals from radiopharmaceutical production and handling through patient dosing, imaging, diagnosis and therapy with our line of dose calibrators, thyroid uptake systems, shielding systems, management software and supporting accessories.
Radiotherapy (also known as radiation therapy or radiation oncology), uses radiation in the form of X-rays, protons and electrons to destroy cancer cells and shrink tumors. We provide both hardware and software products, as well as services to accomplish the critical task of performing independent quality management in the diagnosis and treatment of cancer. Our suite of patient, machine and diagnostic QA solutions are relied on in the field to mitigate errors, reduce inefficiencies, validate technologies and techniques, and elevate the quality of clinical care.
Medical imaging encompasses a number of technologies (MRI, Ultrasound, X-ray) that are used to view the human body to diagnose, monitor or treat medical conditions. We provide support for these imaging techniques through our array of C-Arm and ultrasound tables, MRI accessories, positioners and radiation protection accessories.
As a result of the proliferation of radiological medical technologies, hospitals, clinics, and small dental and veterinary facilities rely on occupational dosimetry systems and services to ensure the safety of both medical personnel and patients. Our dosimetry services products like Instadose, provide instant dose measurement results when connected to any computer or mobile device via Bluetooth and ensure that radiation safety programs run smoothly and are easy to administrate.
Laboratories and Research
The laboratory and research market includes different types of facilities like environmental radiochemistry laboratories, research laboratories, research reactors and education laboratories. All these facilities analyze nuclear samples or monitor experiments to identify the chemical composition of the material involved or understand the basic structure of matter.
The environmental radiochemistry laboratories, or counting labs, monitor the environment by analyzing samples, measuring their radiation and identifying the source and the nature of contamination, if any. The laboratories can be governmental (e.g., health or environment institutions, safety authorities) or private (e.g., facility bio assay, process labs). We believe there are over 500 environmental laboratories worldwide based on our estimates as of December 2021.
Research centers include national laboratories and research institutions conducting research in the areas of space, underground studies, physics around synchrotrons and accelerators. Radiation measurement systems are used in research for the discovery of elements, to study the formation of matter after the big bang or in the deepest underground laboratories in the world to perform dark matter experiments. They are also used in space, mounted in satellites or robots, landing on planets (Mars Rover) or orbiting around Earth (STEREO), Saturn (Cassini), Venus and Mercury (Messenger), Pluto (New Horizons), Mars (MSL-Rad), and Jupiter (JUNO).
Research reactors are used for research and training, materials testing, medicine (like the production of radioisotopes) and industrial functions. According to the WNA, there were 220 operational nuclear research reactors in 53 countries, with 11 more under construction and 16 planned to be built, as of June 2021.
Education laboratories are located in universities and offer programs in nuclear engineering, health physics, radioprotection, nuclear physics or nuclear science and technology. We believe there are more than 600 colleges worldwide, universities and degree-granting institutions that are equipped with nuclear measurement products.
Nuclear
The nuclear end market spans the entire nuclear fuel cycle, including mining, enrichment, fuel manufacturing, nuclear power generation, waste management and fuel reprocessing. Key nuclear installations include mines, fuel fabrication facilities, commercial nuclear power reactors, reprocessing facilities, research facilities, military facilities and ships, weapons facilities and waste storage facilities.
We sell products and services for use in each of these types of installations at any stage of their life (construction, operation, decommissioning and dismantling), with commercial nuclear power reactors representing the majority of our sales into the nuclear end market. This market is segmented between new builds, installed base requesting upgrades/uprates/relicensing, and decommissioning and dismantling.
Driven by increasing demand for electricity and reliable and carbon-free energy, the nuclear power market is forecasted to grow in the near and long term, which presents opportunities for us. These trends are further driven by global decarbonization goals, which are likely to increase the demand for nuclear power.
Despite some challenges in certain regions of the world (e.g., Germany, Sweden, Japan), the new build market is expected to be very dynamic. According to the WNA, in January 2022 there were 57 reactors in construction and 423 planned or proposed. The installed base market presents opportunities while nuclear power plants, or NPPs, are being relicensed with extended life time and upgraded. Meanwhile, we believe that more than 50 reactors will be shut down by 2030, growing the demand for radiation equipment used in decommissioning and dismantling of nuclear facilities.
Defense
Our global defense end market is driven by a combination of military, civil defense and event-driven security spending. The proliferation of global security threats has reached an unprecedented level, driven by an unstable geopolitical climate, the emergence and expansion of terrorist organizations, the development of nuclear weapons in non-nuclear countries and the proliferation of radiological and nuclear technologies. Taken together, these threats have the potential to cause significant human casualties and economic loss. As a result, militaries, civil defense and other security organizations have bolstered investment in the prevention and detection of radiological threats as well as in technologies capable of detecting and monitoring radiation levels in the aftermath of radiological attack.
Militaries throughout the world utilize radiation detection technologies for troop security. Spending on personnel protection and detection of radiological threats is a priority for both NATO and non-NATO militaries and, as such, has led many countries to provide dosimeters to military personnel on a standard-issue basis. We believe that spending on these technologies will remain a high priority among armed forces globally.
Spending within the global civil defense, or homeland security, market has rapidly expanded in recent years based on increased threats presented by terrorist organizations. As a result, civil defense, first responder and other security organizations are investing in technologies and services designed both to protect civil defense personnel, civilians and domestic infrastructure from radiological threats and to detect and monitor radiation levels following a radiological incident, such as the release of a nuclear or other radiological device.
In addition, homeland security organizations are increasingly focused on enhancing radiological detection capabilities at critical points of entry, such as airports, ports and borders. Large-scale public meeting events have also greatly increased security measures at facilities, including rapid adoption of radiological detection technologies to address the increased threat of radiological attacks, due to their profile as high visibility targets.
Industrial
Other end markets include industrial facilities such as cement kilns, pulp and paper mills and coal/gas fired power boilers that utilize high-temperature industrial processes. These high-temperature processes are critical to plant operation and must be accurately monitored to ensure optimal operating conditions. Imaging equipment capable of withstanding the high temperatures and environmental conditions found in these facilities is employed to monitor and optimize process efficiency. These imaging systems require routine replacement or upgrades.
Other end markets also include original equipment manufacturers, or OEMs for general industrial market or medical applications, using radiation measurement detectors to sort material or precisely locate some radioisotopes.
Our Market Opportunity
We believe that significant opportunities for growth exist within each of our primary end markets.
Medical
Radiological procedure growth. The use of radiodiagnostic and radiotherapeutic procedures is expanding globally due to aging population demographics, technological advancements and emerging middle classes in developing economies. As the use of radiological procedures increases in the medical industry, so does our associated market opportunity. According to a global consulting firm, we believe the global nuclear medicine market is expected to grow approximately 7% per year from 2020 through 2030, primarily driven by the increase in the prevalence and incidences of cancer worldwide. Likewise, the global radiotherapy market is expected to grow approximately 6% per year from 2020 through 2030, primarily driven by factors including growing awareness about the benefits of radiotherapy for cancer control and eradication, increasing incidence and prevalence of cancer, and technological advancements in the field of radiotherapy. We play in select sub-segments of the global nuclear medicine and radiotherapy markets. The growth trajectories in these markets represent significant market opportunities for our products that are deployed in hospitals, clinics, and other diagnostic and therapeutic centers around the world.
Dosimetry outsourcing. In some regions outside the United States, dosimetry services for health care practitioners historically have been provided by government agencies. We believe that more government agencies are outsourcing dosimetry services to private providers due to favorable cost dynamics in some regions. This provides a market opportunity where we can leverage our technical expertise and North American service experience to expand into other regions as we have done through our acquisitions of state-owned dosimetry services businesses in the Netherlands and Germany. According to a global leading consulting firm, we believe our core dosimetry market is expected to grow 3 to 4% per year from 2020 through 2026, primarily driven by volume increase in number of healthcare workers exposed to radiation and standard annual price increases. In addition, through the differentiating factors behind the innovative Instadose product line, we believe that we have the right product ecosystem to maximize this opportunity.
Laboratories and Research
Customer loyalty. Loyalty is driven by long standing relationships, customer hesitancy to switch suppliers, high switching costs and limited competition globally. We believe we can benefit from price growth in most of our markets. In addition, our business is well protected by consistent replacement cycles on installed base.
Nuclear
Our legacy in the nuclear industry positions us to capitalize on the growth in demand for radiation detection, measurement, analysis and monitoring products and services in each phase of the nuclear life cycle, as outlined in the chart below.
We provide essential products and services to NPPs throughout the entire life cycle of a plant: from construction and operation to decommissioning and decontamination. For example, we provide:
•Radiation measurement and monitoring solutions, such as detection portals, environmental monitors and dosimetry systems that are typically installed in nuclear facilities during construction and are replaced or upgraded during the entire lifetime of the reactors, in particular upon life extensions. This provides recurring revenue opportunities as customers must replace and upgrade components and services during this timeframe,
•Reactor instrumentation and control detectors that are typically installed in nuclear facilities during construction and are replaced or upgraded regularly. In addition, there are opportunities to provide more comprehensive upgrades of reactor instrumentation and control detector systems in certain existing reactors to facilitate up-rating,
•Measurement and expertise services including technical expertise and experienced staff to help customers address their nuclear measurement needs in every step of the measurement process from planning to operation to wind-down,
•Imaging systems and cameras for all stages of the nuclear lifecycle, from construction through operation, to decommissioning and waste management, and
•Waste management systems that are used during the lifetime of the reactors and are essential, in particular, in any decontamination and decommissioning project.
We believe the following dynamics support the sustainability of our existing business and will drive new sources of organic growth.
Predictable upgrade, replacement and retirement cycles. Our radiation detection, measurement, analysis and monitoring products and systems have predictable life spans, typically ranging from four to twenty-five years. Our complex monitoring systems typically require at least one comprehensive upgrade during their useful life to optimize their functionality. In addition, many of our products require replacement parts, components and service due to normal wear during their useful lives.
Aging installed base. The existing global installed base of nuclear reactors has a median age of 34 years. This aging installed base requires frequent product replacements and upgrades over an operating life cycle that generally ranges from 40 to 80 years. Furthermore, as reactors reach the end of their useful lives, the onset of a multi-year “decommissioning” process represents a further revenue opportunity in the reactor life cycle for our products.
Increased decontamination and decommissioning activity and stricter environmental regulation. The total number of NPP shutdowns under decontamination and decommissioning is expected to increase over the next decade, with largest amount of expected plant shutdowns potentially in the U.S. market. In Europe, the UK represents the highest share of expected shutdowns as the operating fleet ages and passes the license extension period.
Large installed base of “orphaned” products and systems. Most currently operating reactors were commissioned prior to 1990. Operators of many aging NPPs often must consider new suppliers to meet their detection needs as many of the suppliers of legacy radiation detection, measurement, analysis and monitoring systems no longer service the nuclear industry.
Dosimetry outsourcing. NPPs have historically managed the majority of their dosimetry service requirements internally. However, the cost benefits of outsourcing these services have become increasingly attractive to NPP operators as they focus on improving profitability and enhancing service.
New build opportunity. We expect the construction of new nuclear reactors worldwide to provide opportunities across our product and service offerings. The nuclear industry is experiencing robust growth in activity related to new reactor builds. According to the WNA, in January 2022, there were 57 reactors under construction and 423 planned or proposed. This growth is occurring internationally and our global footprint positions us to capitalize on these opportunities. Since the early stages of reactor development generally represent a material share of our revenue opportunity over the life cycle of a reactor, we are positioned to benefit from increased global reactor construction. In addition, as new plants are added to the global nuclear fleet, we believe our recurring revenue opportunity associated with replacements, spares, software, services and system upgrades will continue to increase as we are well-positioned with customers due to our incumbent position.
Defense
Focus on military personnel. Global militaries must contend with radiological threats and the difficulties of protecting soldiers and monitoring areas of enemy engagement. The combination of our active dosimeters and telemetry technology provides a differentiated solution that addresses the radiation detection needs of modern militaries.
Increased civil defense spending on radiation detection. Civil defense and homeland security organizations are focused on preventing the illicit transportation of radiological materials across borders. The commercial application of our radiation detection expertise positions us to benefit from government spending on detection technologies.
Enhanced event specific security. The visibility of high profile events and venues has increased their value as targets of terrorist activity. In response, security spending at events, such as the Olympic Games, has increased, as has the utilization of radiation detection technology, providing an expanding market opportunity for our products.
Our Competitive Strengths
We believe that the following competitive strengths will enable us to maintain our position and capitalize on growth opportunities in our end markets:
Trusted ionizing radiation detection and measurement provider. Our end markets, including the medical, defense and nuclear industries, are highly regulated and require compliance with strict product specifications. Our track record enables
us to gain market share across our product and service offerings. We and our predecessor companies have served the radiation detection measurement, analysis and monitoring needs of our customers for over 60 years, having developed trusted, recognized brands supported by our tradition of technical excellence, product reliability and customer service. We believe we have a leadership position in 14 of the 17 market segments we serve. In addition, we have leveraged our ionizing detection expertise to develop new applications for our core historical markets and to expand into adjacent markets through acquisitions.
Broad and complementary product and service portfolio. We are one of relatively few companies to offer ionizing radiation detection and measurement products and services to satisfy customer requirements throughout the medical and industrial markets. Our comprehensive product line supports virtually all radiation detection and monitoring needs associated with these markets. As a result, we believe that we have consistently gained market share as some of our key customers rationalize their supply chain. Furthermore, our portfolio provides us with a natural opportunity to cross-sell our products and services to our customers. As a result, we have a diversified portfolio across end markets and geographies.
Large installed base driving recurring revenue. We possess longstanding customer relationships in all of our end markets. We believe our QA products are used by the vast majority of cancer treatment centers in the United States and in the majority of such centers globally. This drives recurring revenue and opportunities for cross sales from our other activities. Our products were also installed at the vast majority of the addressable installed base of active nuclear power reactors globally, which have a median age of about 34 years. This installed base drives recurring revenue through replacement and service cycles associated with our offerings and the typical 40 to 80 year operating life cycle of an NPP. The length and quality of supplier relationships are important customer buying criteria due to high switching costs and the importance of proven product reliability. In addition, we maintain relationships with global military and government organizations that value operating longevity and technological expertise. For example, our products have been sold to 19 of the 28 NATO militaries as well as the U.S. Departments of Energy, State, Defense and Homeland Security. Our customers’ focus on personnel protection drives their recurring expenditures on service, recalibration and product upgrades in our defense end market. In the laboratories and research markets, we have developed relationships with certain customers over the past 50 years, gaining their loyalty based on product performance and customer services. Such relationships provide us with recurrent revenues when our customers upgrade and replace their existing installed base.
Technical complexity creates high barriers to entry. Across our end markets, we design our products to meet demanding customer specifications, qualifications and regulatory requirements. In many circumstances, we design our products to be compatible with highly complex facilities and operate effectively in harsh environments. Replicating our products is difficult given underlying technical specifications. In addition, customers generally work with their incumbent suppliers to service, maintain and replace equipment over product lifetime resulting in a natural barrier to entry.
Global footprint designed to meet local customer needs. Our global footprint, augmented by our established network of suppliers and distributors, enables us to be responsive to our customers and provide locally customized solutions. We operate facilities in 12 countries, accommodating the desire of certain of our customers to procure products and services from local providers. Sales to customers outside of the United States and Canada accounted for approximately 45% of total revenue for fiscal 2021. We believe that our established global infrastructure provides a scalable platform to meet the growing worldwide demand for our products and services.
Proven M&A strategy and track record of integrating acquisitions. We have been built through successive mergers and acquisitions. Since 2016, we have acquired and integrated fourteen companies. Through these acquisitions, we have developed tools and experience across deal sourcing, modeling and integrating acquired companies. We have a business ecosystem in place to identify and act upon cost saving opportunities as well as the ability to leverage our scale platform to capture cross-selling opportunities. Historically, we have consistently exceeded our synergy targets and improved profitability of acquired businesses.
Seasoned management team complemented by highly skilled engineers. We are led by an experienced management team with a mix of private sector and government experience across different industries and functions. Our segment and divisional presidents have an average tenure of over 15 years. Our senior management team is complemented by an engineering and research and development organization of 356 scientists, engineers and technicians as of December 31, 2021. A number of our employees are participants in international and U.S. standards setting organizations related to radiation detection in the nuclear, defense and medical end markets. Through these activities, we help define the setting of standards and preview changes that impact our products, customers and end markets.
Our Strategy
Our objective is to continue enhancing our position as a global provider of radiation detection, measurement, analysis and monitoring products and services for the global medical and industrial end markets. We intend to achieve this through the following strategies:
Exploit under-penetrated market opportunities. We believe that we can exploit historically under-penetrated segments of our end markets by leveraging our existing positions across our major product categories. For example, we have leveraged our technical expertise to develop and commercialize innovative products to increase sales in the U.S. dosimetry services market and in the radiotherapy quality assurance market, and we have expanded our radiation monitoring solutions offering by leading integrated offers with other key suppliers for some nuclear new build projects in Europe to increase our scope of supply and gain share in the nuclear market.
Expand addressable market. We believe that substantial opportunities exist for us to expand our addressable market by marketing our products and services to customers in new geographic regions; providing products and services to customers moving to an outsource model; entering markets where the government is privatizing services; introducing new applications for existing technologies and pursuing strategic acquisitions.
•Geographic expansion. Although we have sold products and services to customers in 120 countries historically, we believe we have additional opportunities in certain international markets. For example, in India, a market we currently serve through local partners, we intend to leverage our relationships with leading reactor design firms to capitalize on the opening of the nuclear end market to U.S. and European firms. Another such market is the European dosimetry services market. Through acquisitions, we have developed our presence in the Netherlands and Germany, and we plan to continue expanding into other European countries. Other markets for expansion include the Middle East, Eastern Europe and the former Soviet Union, where we intend to increase our presence by leveraging relationships with local partners.
•Customer outsourcing. We believe we will continue to capitalize on customer outsourcing within the nuclear end market. Within the United States, several NPP operators have recently outsourced their dosimetry services in order to reduce costs. We have been able to benefit from economies of scale as well as advantages in materials procurement and processing technology to provide enhanced dosimetry services to many of these NPPs at a lower cost.
•Service privatization. In regions outside the United States, dosimetry services have historically been provided by government agencies. However, privatization of dosimetry services is occurring in some regions, such as Europe. As illustrated by our acquisitions in the Netherlands and Germany, providers seek to reduce costs and benefit from enhanced service offerings. This provides us with an opportunity to leverage our expertise and North American service experience, where we have demonstrated a strong track record of success, to expand market share in other geographies.
•Expand into new end markets. We periodically review our adjacent markets and identify opportunities for expansion. For example, we have developed a new personal radiation detector, or PRD, called Accurad to expand our presence in the civil services markets such as the police and fire departments. We have also entered in the nuclear imaging and radiotherapy markets through the acquisitions of Capintec, Biodex and Sun Nuclear, and, most recently in December 2021, CIRS.
•New applications for existing technologies. A portion of our development effort is focused on adapting existing technologies to alternative applications. For example, we have adapted the technology used for the medical and nuclear markets to develop the Mirion Battlefield Dosimeter which is currently being deployed by the U.S. Army and the U.S. Navy.
Develop new products and services. We believe that significant near-term opportunities exist for us to develop new products and services by capitalizing on our understanding of our customers’ needs and requirements. Cross pollination of technologies between end markets also drives new growth opportunities. For example, we created a new product called evrCAM to meet the needs of the radiation oncology market by leveraging our core technology from decades of experience in radiation tolerant cameras for the nuclear power industry.
Continuously improve our cost structure and productivity. As we continue to grow our business, we have implemented a coordinated program of ongoing operating improvements, such as optimizing our manufacturing footprint, rationalizing excess costs and minimizing working capital requirements. We are continuously implementing our business system principles to challenge our practices and improve our performance across all our businesses. For example, we have
optimized and simplified our footprint by transferring the activities from our facilities in Loche, France, certain activities in our Irvine, California facility and certain activities in our Shirley, New York facility to other Company sites. Our global procurement team also delivers value across the business from sourcing of key materials and services to supply chain design.
Pursue strategic acquisitions. We have successfully integrated acquisitions to augment our organic growth. We were formed by the merger of Global Dosimetry Solutions, or GDS, Imaging and Sensing Technologies, or IST, and Synodys in 2005. In 2016, we acquired Canberra Industries. Between October 2018 and December 2021, we acquired twelve companies, with the objective of complementing our portfolio, reinforcing our supply chain and expanding into new markets such as nuclear imaging and radiotherapy. Since then, we have effectively integrated these businesses, creating a global platform of ionizing radiation detection and measurement solutions. We continuously monitor potential acquisitions and intend to further complement our organic growth with selective acquisitions that enhance our existing products and services, strengthen our position with existing customers and enable us to expand into new markets.
Our Segments
Medical
Our Medical segment encompasses five major product categories focused on supporting applications in medical diagnostics, cancer treatment, practitioner safety and rehabilitation. Our products in these fields are focused on enhancing the effectiveness and safety of life-saving procedures.
•Cancer Diagnostics and Therapeutics QA: we provide integrated solutions for independent quality management in the diagnosis and treatment of cancer. Our suite of patient, machine, and diagnostic QA solutions are relied on in the field to mitigate errors, reduce inefficiencies, validate technologies/techniques and elevate the quality of clinical care. Our products include arrays for machine and patient QA solutions, software platforms for centralized analysis and data storage, lasers to align Linacs to patient or QA devices, and phantoms (devices to simulate the imaging and radiation dose absorption characteristics of human tissue) for machine and patient QA.
•Nuclear Medicine and Medical Imaging: we provide solutions for patient dosing, imaging, diagnosis and radiopharma production and handling as well as specialized medical imaging tables and accessories that support imaging techniques and procedures. Our products include our range of dose calibrators, radiation shielding, phantoms for quality assurance, phantoms, thyroid uptake systems, lung scan ventilation systems, ultrasound tables, C-Arm tables and accessories.
•Medical Imaging: we provide specialized medical imaging tables and accessories that support imaging techniques and procedures, including ultrasound tables, C-Arm tables and accessories.
•Dosimetry Services: our product offering is an information service, which provides environmental radiation monitoring services, as well as an official dose of record to employers and occupationally exposed employees, enhancing the effectiveness and efficiency of radiation safety programs at practitioner sites. Key product lines include the innovative Instadose dosimetry platform, optically stimulated luminescence, or OSL, dosimeters, and our range of eye, finger, and extremity dosimeters that integrate with our Dose Central data platform.
•Rehabilitation: we provide neuromuscular assessment and rehabilitation technology solutions. Our products are used to manage and rehabilitate the physical and performance deficits that cause functional limitations. Our technology safely progresses a patient through the physical rehabilitation progress. Our rehabilitation products are used in patients throughout the continuum of life – from injuries requiring sports medicine and orthopedics to interventions for our aging population such as fall prevention and all ages with neurologic conditions due to strokes, Parkinson’s Disease, spinal cord and traumatic brain injury. Our products include isokinetic testing and rehabilitation systems, balance assessment and rehabilitation, specialized gait training treadmills, body weight support training systems and upper, lower and total body ergometers.
Industrial
Our Industrial segment is focused on addressing critical radiation safety, measurement and analysis applications across defense, nuclear energy, laboratories and research and other industrial markets.
Reactor Safety and Control Systems: we provide radiation monitoring systems and reactor instrumentation and control systems that ensure the safe operation of nuclear reactors and other nuclear fuel cycle facilities. Product lines include, but
are not limited to, a range of areas such as effluent release and operational process monitors, as well as in-core and ex-core detector systems, electrical penetrations, boron meters, and nuclear containment seals. Select product categories include:
•Radiation Monitoring Systems: sensors, displays, control electronics and software used for barrier leak control, effluent release monitoring, operational process monitoring and “post event” monitoring in NPPs, nuclear fuel cycle industry, research reactors and laboratories, military reactors and installations.
•Reactor Instrumentation and Control Equipment and Systems: sensors, cables and electronics designed to monitor radiation and temperature within a reactor core and in surrounding areas.
•Neutron Flux Measurement Systems: sensors, displays, control electronics and software used to control the core of a reactor in NPPs, research reactors, and military reactors.
Radiological Search, Measurement and Analysis Systems: we provide solutions to locate, measure and perform in-depth scientific analysis of radioactive sources for radiation safety, security, and scientific applications. Product portfolios include but are not limited to our laboratory and scientific analysis systems (gamma/alpha spectroscopy, alpha/beta counting, specialty detectors, spectroscopy software), radiation measurement and health physics instrumentation (contamination and clearance monitors, portable radiation measurement, electronic dosimetry, telemetry, waste measurement) and search and radiological security systems (Military CBRNE, or Chemical, Biological, Radiological, Nuclear and high-yield Explosives, security and search). We also provide a wide range of on-site managed and professional services to our end market customers. Select product categories include:
•Dosimeters: active and passive dosimeters which monitor radiation dose rate and cumulative dose, along with readers, calibrators, telemetry, software and other accessories.
•Contamination and Clearance Monitors: stationary systems designed to detect radioactive contamination of people, waste, tools, laundry, vehicles and cargo.
•Detection & Identification Devices: hand-held and fixed devices to detect and locate ionizing radiation.
•Customized Research Detectors: highly customized detectors for scientific research, including nuclear physics research, space and synchrotron applications, and ruggedized detectors.
•Environmental Monitoring Systems: sensors, displays, control electronics and software used for environmental monitoring in NPPs, nuclear fuel cycle industry, research reactors and laboratories, military reactors and installations.
•Radiochemistry: high precision instruments for detection and analysis of sample radioactivity, identification of radionuclide and quantification of activity used in laboratories, research, education, defense and NPPs.
•Imaging Systems: radiation-hardened imaging systems for nuclear fuel handling, control, monitoring and inspection; reactor vessel maintenance; underwater surveillance; tank and vessel inspection; and cameras for remotely operated vehicles.
•Waste measurement systems: systems to measure the radioactivity content of waste such as gamma neutron counting systems, non-destructive assay systems and neutron counting systems
•Services: we offer services to measure and analyze nuclear material more efficiently, calibration services, customer training programs, installation of instruments and software, technical support and repairs for our products, as well as local operational support, technical support, and a wide range of consulting services
Backlog and Deferred Contract Revenue
Total backlog represents committed but undelivered contracts and purchase orders at period end. Backlog excludes maintenance-related activity and agreements that do not represent firm purchase orders. Customer agreements that contain cancellation for convenience terms are generally not reflected in backlog until firm purchase orders are received. Backlog is not a complete measure of our future business due to these customer agreements. Our customers may experience project or funding delays or cancel orders due to factors beyond our control. If customers terminate, reduce or defer firm orders, whether due to fluctuations in their business needs or purchasing budgets or other reasons, our sales will be adversely affected and we may not realize the revenue we expect to generate from our backlog or, if realized, the revenue may not
translate into profit. We estimate approximately 10%-15% of our backlog at any point in time is related to contracts that are unfunded and may be at risk for cancellation if funding is not appropriated. Backlog can fluctuate significantly due to the timing of large project awards. In addition, annual or multi-year contracts are subject to rescheduling and cancellation by customers due to the long-term nature of the contracts.
Deferred contract revenue represents prepayments from customers, including milestone or installment payments, on projects for which services have commenced, as well as unbilled amounts attributable to services rendered and products constructed associated with customer contracts for which revenue is not able to be recognized.
Information on backlog and deferred contract revenue follows (in millions):
| | | | | | | | | | | | | | |
| Successor | | | Predecessor |
| December 31, 2021 | | | June 30, 2021 |
| Backlog | $ | 747.5 | | | | $ | 715.8 | |
| Deferred contract revenue | $ | 73.0 | | | | $ | 50.4 | |
Approximately 45% of our backlog is expected to be recognized in calendar year 2022.
Competition
The global markets for our products and services are competitive and continually evolving. Within each of our operating segments, we encounter a variety of competitors, ranging from small independent companies providing niche solutions to larger multi-national corporations providing a broader set of products and services to our targeted end markets. We believe that the principal bases upon which we compete in our target end markets include product quality and reliability, technical capability and product qualification, strength of customer relationships, customer service and price. In particular, customers in the defense and nuclear end markets tend to emphasize product quality and reliability, technical capability and strength of supplier relationships, while customers in the medical end markets, in particular for passive dosimetry products and services, tend to make purchasing decisions based on a combination of brand recognition, price, service and reliability.
We believe the primary competitors in each of our segments are as follows:
•Medical: Landauer (Fortive), PTW, IBA, Standard Imaging, Comecer and LAP
•Industrial: Thermo Fisher Scientific, Ortek (Ametek), FLIR (Teledyne), Framatome, Ludlum, Fuji Electric, Caen System, Fluke (Fortive) and Berthold Technologies
Research and Development
Our research and development efforts allow us to introduce new products to the marketplace, fulfill specific customer needs and continue to meet qualification requirements and other evolving regulatory standards. Our Medical and Industrial segments are committed to both technology research and product development to fulfill their strategic objectives and are supported by our engineering and research and development organization consisting of 356 scientists, technicians and engineers, representing approximately 14% of our total workforce, as of December 31, 2021. A number of these individuals participate in international standards setting organizations and committees. We engage in research and development activities at most of our facilities worldwide.
Our research and development expenses were $6.7 million for the Successor Period from October 20, 2021 through December 31, 2021, $10.3 million for the Predecessor Stub Period from July 1, 2021 through October 19, 2021, and $29.4 million, $15.9 million and $14.0 million for fiscal 2021, 2020 and 2019, respectively. We conduct these efforts through a mix of in-house research, collaboration with academia, customers and regulatory authorities as well as selected outsourcing through external vendors. The scope and extent of the outsourced portion of research and development activities vary by segment but typically, critical hardware design, software development and project management activities are conducted in-house while specialized services such as consulting services, algorithm design, thermal analysis, complex modeling and calculations and testing services are provided by third parties.
Sales and Marketing
We sell our products and services through our direct sales organization and indirectly through our global network of independent, third-party sales representatives and distributors. Our internal sales team is organized by operating segment and end market to provide a higher level of service and understanding of our customers’ unique needs. We have 30 sales
offices throughout North America, Europe and Asia, and as of December 31, 2021, our sales and marketing personnel consisted of 248 employees, which represents approximately 9% of our total workforce.
We derive a portion of our revenue from sales of our products and services through channel partners, such as independent sales representatives and distributors. In particular, our independent sales representatives are an important source of sales leads for us and augment our internal resources in remote geographies. We sell through distributors in situations in which our customers prefer to purchase from a local business entity or purchase in smaller volume.
Our marketing activities include participation in many trade shows worldwide across our defense, medical and nuclear end markets. We advertise in technical journals, publish articles in leading industry periodicals and utilize direct mail campaigns.
Except when prevented by exceptional circumstances (for example, the COVID-19 crisis), we periodically host seminars and participate in trade shows. For example, we host the annual Mirion Connect Seminar, where customers participate in a variety of programs designed to exchange ideas and discuss occupational challenges. The event also brings together key channel partners and vendors to strengthen our sales and marketing network. Attendees gain insight into our product plans and participate in interactive sessions that give them the opportunity to better understand our current suite of products and services as well as provide feedback on our product roadmap.
Our Customers
Our principal customers include hospitals, clinics and urgent care facilities, dental offices, veterinary offices, radiation treatment facilities, OEMs for radiation therapy, laboratories, military organizations, government agencies, industrial companies, power and utility companies, reactor design firms and NPPs. We have long-standing relationships with our customers. For the Predecessor Stub Period from July 1, 2021 through October 19, 2021 and the Successor Period from October 20, 2021 through December 31, 2021 no customer accounted for greater than 6% of our consolidated revenue, our top five customers together accounted for approximately 14% and 13% of our consolidated revenue, respectively, and our top ten customers represented approximately 20% and 19% of our consolidated revenue, respectively.
Manufacturing and Supply Chain
Given the diversity of our products, we employ numerous manufacturing techniques, including high-volume process manufacturing, discrete manufacturing, cellular manufacturing and hybrid approaches. Our production personnel engage in manufacturing, procurement and logistics activities. Our production activities are located in the United States, Canada, France, Germany, Belgium, Estonia, Finland and the United Kingdom. As of December 31, 2021, our production personnel consisted of 1,176 employees, which represents approximately 45% of our total workforce.
Our manufacturing activities are focused mainly on the production of the core value-add devices and components of our products, while non-core components and sub-assemblies are generally outsourced. This strategy enables us to protect important intellectual property and trade secrets while minimizing the time, cost and effort to produce commoditized components. Most of the time, the design, assembly and integration of the components are performed in-house, allowing our engineers to customize the products according to customer specifications. For highly engineered nuclear products, production volumes are typically low. For other product lines, such as, the DMC 3000 Electronic Dosimeter, the Mirion Battlefield Dosimeter, Accurad PRD and the Instadose dosimeter, production volumes tend to be higher. We apply rigorous quality control processes and calibrate radiation detection devices internally, leading to high quality standards and customization capabilities. Most of our production sites are certified to production quality standards such as those of ISO 9001, the U.S. Nuclear Regulatory Commission (10 C.F.R. 50 Appendix B) and the American Society of Engineers (ASME NQA-1).
The principal materials used in our manufacturing processes are commodities that are available from a variety of sources. The key metal materials used in our manufacturing processes include precious metals, tungsten, copper, aluminum, magnesium products, steel, stainless steel and various alloys, which are formed into parts such as detectors, sensors, metal housings and frames, and cable assemblies. The key non-metal materials used in our manufacturing processes include amorphous and crystalline scintillator materials, ceramics, epoxies, silicon and fused silica, polyethylene, polyurethane and injection molded plastic parts and components such as lenses, monitors, sensors, dosimeters, electronic boards, detectors and cables.
Human Capital Resources
As of December 31, 2021, we employed 2,630 full-time and part-time employees. We also use temporary or contract workers who totaled approximately 115 as of December 31, 2021, on a full-time equivalent basis.
Diversity and Inclusion
We are committed to fostering a diverse and inclusive workplace that attracts and retains exceptional talent. We value teamwork, practicing intellectual honesty and candor, with a clear focus on the situation, not the individual. We support a diversity of backgrounds, experiences and perspectives in our workforce and promote an engaging workplace that
encourages participation and inclusion of all employees.
Total Rewards Compensation Philosophy
We require a talented workforce and are committed to providing total rewards that are market-competitive and performance-based, driving innovation and operational excellence. Our compensation programs, practices and policies reflect our commitment to reward short- and long-term performance that aligns with, and drives, stockholder value. Total direct compensation is generally positioned within a competitive range of the relevant market median, with differentiation based on tenure, skills, proficiency, and performance.
Employee Engagement
We regularly collect employee feedback to better understand and improve employees' experience and identify opportunities to continually strengthen our culture and communicate through town halls, emails and other communication platforms. We mandate quarterly check-ins between employees and their managers as key human capital measures and objectives. We want to know what is working well, what we can do better and how well our employees understand and are practicing our cultural values.
Training and Development
Human capital development underpins our efforts to execute our strategy and continue to design, manufacture and market innovative products and services. We continually invest in our employees’ career growth and provide employees with a wide range of development opportunities, including but not limited to mentoring, product and sales training, and compliance training.
Intellectual Property
The success of our business depends, in part, on our ability to maintain and protect our proprietary technologies, information, processes and know-how. We rely on a combination of intellectual property rights, including trade secrets, patents, copyrights and trademarks, as well as contractual protections, to protect our proprietary products, methods, documentation and other technology.
As of December 31, 2021, we own approximately 75 issued U.S. utility patents, 66 issued foreign utility patents (including in Canada, the European Union, Russia, China and Japan), 10 pending U.S. utility non-provisional patent applications, 26 pending foreign utility patent applications (including in the European Union and France) including pending Patent Cooperation Treaty, or PCT, patent applications. These issued patents are expected to expire between 2022 to 2038 and these pending applications, if issued, are expected to expire between 2039 to 2040, in each case without taking into account any possible patent term adjustment or extensions and assuming payment of all appropriate maintenance, renewal, annuity, or other governmental fees. We do not expect the expiration of any of the patents that are scheduled to expire in 2022 to have a material impact on its business. These patents include one co-owned issued U.S. patent and three co-owned issued foreign patents. We also hold exclusive and non-exclusive licenses related to patents and other intellectual property of third parties. We also own trademark registrations or registration applications in the United States and in certain foreign jurisdictions.
Medical Segment
As of December 31, 2021, we own approximately 38 issued U.S. utility patents, 23 issued foreign utility patents (including in the European Union, China, Japan and Canada), 8 pending U.S. non-provisional utility patent applications and 6 pending foreign utility patent application in the European Union that include claims directed to products in our medical segment, including our cancer diagnostics and therapeutics QA, occupational dosimetry, medical imaging and nuclear medicine equipment products. These issued patents are expected to expire between 2022 to 2038 and these pending applications, if issued, are expected to expire between 2039 to 2040, in each case without taking into account any possible patent term adjustment or extensions and assuming payment of all appropriate maintenance, renewal, annuity, or other governmental fees.
Industrial Segment
As of December 31, 2021, we own approximately 37 issued U.S. utility patents, 43 issued foreign utility patents (including in the European Union, Canada, Russia and Japan), 2 pending U.S. non-provisional utility patent application and 20 pending foreign utility patent applications (including pending PCT patent applications) that contain claims directed to products in our industrial segment, including our alpha/beta counting instruments, contamination and clearance monitors, gamma spectroscopy software and detector systems, NDA and waste measurement systems, portable radiation measurement instruments, radiation monitoring systems and reactor instrumentation and controls products. Our issued patents are expected to expire between 2022 to 2037 and our pending applications, if issued, are expected to expire between 2032 to 2040, in each case without taking into account any possible patent term adjustment or extensions and assuming payment of all appropriate maintenance, renewal, annuity, or other governmental fees. These patents include one co-owned issued U.S. patent and three co-owned issued foreign patents.
In many instances (for both the Medical and Industrial Segments), we rely on trade secret protection and confidentiality agreements to safeguard our interests. Due to the long useful life of certain aspects of our technology, we believe that the patent registration process, which requires public disclosure of patented claims and inventions, could harm our competitive position. We differentiate our products and technologies primarily through our proprietary know-how, technology or data that are not covered by patents or patent applications, including technical processes, equipment designs, testing and other procedures. Our employees are generally required to assign to us all of the inventions, designs and technologies they develop during the course of employment with us, either through written agreements or by operation of law, depending on the jurisdiction. Where appropriate, we require third parties with whom we deal to enter into agreements with us that address issues of confidentiality and intellectual property. For a discussion of the risks and uncertainties affecting our business related to our protection of intellectual property and other proprietary information, please see “Part I, Item 1A. Risk Factors—Legal and Regulatory Risks.”
Seasonality
General economic conditions impact our business and financial results, and our business experiences seasonal and other trends related to the industries and end markets that we serve. While we believe that we are poised for growth from governmental customers in both of our segments, our revenues and cash flows from government customers are influenced, particularly in the short-term, by budgetary cycles. This impact can be either positive or negative. However, as a whole, we believe we are not subject to significant seasonality. For more information about the trends that impact our business and financial results, see “Part I, Item 1A—Risk Factors—Risks Related to Our Business and Industry—Our results of operations may fluctuate significantly, which could make our future results difficult to predict and could cause our results of operations to fall below expectations."
Environmental Matters
We are subject to a variety of environmental, health and safety and pollution-control laws and regulations in the jurisdictions in which we operate. We use, generate, discharge and dispose of hazardous substances, chemicals and wastes at some of our facilities in connection with our product development, testing and manufacturing activities. In addition, some of our facilities are located on properties with a history of use involving hazardous substances, chemicals and wastes and may be contaminated.
Under the Comprehensive Environmental Response, Compensation and Liability Act of 1980, or CERCLA (also known as the Superfund Law) and its state analogues, we may be subject to joint and several liability for environmental investigations and cleanups, including at properties that we currently or previously owned or operated, or at sites at which waste we generated was disposed, even if the contamination was not caused by us or was legal at the time it occurred. Although we have not incurred any material liabilities in connection with contamination, we may be required to make expenditures for environmental remediation in the future with respect to contamination at our or our predecessors’ former or current facilities or at third-party waste disposal sites under these laws. The Resource Conservation and Recovery Act of 1976 as amended by the Hazardous and Solid Waste Amendments of 1984, or RCRA, provides a comprehensive framework for the regulation of hazardous and solid waste which applies to our operations. RCRA prohibits improper hazardous waste disposal and imposes criminal and civil liability for failure to comply with its requirements. The Toxic Substances Control Act of 1976, or, TSCA provides a comprehensive framework for the management by the EPA of over 60,000 commercially produced chemical substances, some of which are used by our operations. The Clean Water Act regulates the discharge of pollutants into certain waters and may require us to apply for and obtain discharge permits, conduct sampling and monitoring and, under certain circumstances, reduce the quantity of pollutants in those discharges. The Occupational Safety and Health Act, or OSHA provides for the establishment of standards governing workplace safety and health requirements, including setting permissible exposure levels for hazardous chemicals. We must follow OSHA
standards, including the preparation of material safety data sheets, hazardous response training and process safety management, as well as various record-keeping, disclosure and procedural requirements.
Our operations outside the United States are subject to similar, and sometimes more stringent, laws and regulations. For example, an EU directive relating to the restriction of hazardous substances in electrical and electronic equipment, or RoHS directive, and a directive relating to waste electrical and electronic equipment, or WEEE directive, have been implemented in EU member states. Among other things, the RoHS directive restricts the use of certain hazardous substances in the manufacture of electrical and electronic equipment and the WEEE directive requires producers of electrical goods to be responsible for the collection, recycling, treatment and disposal of these goods. China and South Korea and certain other jurisdictions have laws similar to the RoHS and WEEE directives. In addition, the EU has a regulation regarding the registration, authorization and restriction of chemical substances in industrial products, or REACH. REACH and other regulations requires us or our suppliers to substitute certain chemicals contained in our products with substances the EU considers less dangerous. See “Part I, Item 1A. Risk Factors—Legal and Regulatory Risks—We could incur substantial costs as a result of violations of, or liabilities under, environmental laws.”
Regulation
We are subject to a variety of laws and regulations, including but not limited to those of the United States, Canada, the EU, the EU member states and the People’s Republic of China, that impose regulatory systems that govern many aspects of our operations. In addition, these jurisdictions impose trade controls requirements that restrict trade to comply with applicable export controls and economic sanctions laws and requirements, and legal requirements that are intended to curtail bribery and corruption. These laws and regulations apply by virtue of the nature of our industry, end markets and products, as well as the range of potential uses of our products, the origin of the technology incorporated into our products, and the jurisdictions in which we produce and sell our products.
The multi-jurisdictional legal and regulatory environments in which we operate are subject to extensive and changing laws and regulations administered by various national, regional and local governmental agencies both within and outside the United States.
We are a federal government contractor and, as such, we are subject to Executive Order 11246 and other relevant laws and regulations. As part of our compliance obligations, we implement on an annual basis an affirmative action plan and program which, in part, include our good faith efforts to achieve in our workforce full utilization of qualified women and minorities. In addition, we have in place an affirmative action plan with respect to disabled individuals, as well as Vietnam era, disabled or other veterans.
Some of the U.S. laws affecting our operations include, but are not limited to, the Atomic Energy Act, or "AEA", the Energy Reorganization Act of 1974, or "ERA", as well as the state laws governing radiation control in the states of New York, Georgia, California, Connecticut, Tennessee, New Jersey, Florida and Wisconsin, each as from time to time amended. We are also subject to a variety of U.S. federal and state employment and labor laws and regulations, including the Americans with Disabilities Act, the Federal Fair Labor Standards Act, the Worker Adjustment and Restructuring Notification Act, or "WARN Act", which requires employers to give affected employees at least 60 days’ notice of a plant closing or mass layoff, and other regulations related to working conditions, wage-hour pay, overtime pay, employee benefits, anti-discrimination and termination of employment. We are also subject to the employment and labor laws and regulations of the foreign jurisdictions where many of our employees are located. The classified work that we currently perform at one of our U.S. facilities subjects us to the industrial security regulations of the Department of Defense and other federal agencies that are designed to safeguard against unauthorized access by foreigners and others to classified and other sensitive information.
In the United States, the AEA and ERA authorize the Nuclear Regulatory Commission or "NRC", and state authorities where applicable, to regulate the receipt, possession, use and transfer of radioactive materials. The NRC, and state authorities where applicable, sets regulatory standards for worker protection and public exposure to radioactive materials or wastes to which we are required to adhere in our operations that use radioactive materials in research and development, product manufacture, testing and calibration.
Certain of our products require the use of radioactive sources. For certain of our products, these radioactive sources are often obtained by our customers directly from third-party providers, and for others, we directly incorporate these radioactive sources into our products. Certain of our reactor instrumentation and control equipment and systems for NPPs incorporate radioactive materials. In all such cases, licenses for radioactive sources and materials are provided by the appropriate regulatory authority in the relevant jurisdiction and such authorities may be at the state or national level. For example, at our sites in the United States that handle radioactive sources or materials, the appropriate licenses are issued by state-level authorities which are, respectively, the New York State Department of Health, Georgia Department of Natural
Resources, California Department of Public Health, Connecticut Department of Energy & Environmental Protection, New Jersey Department of Environmental Protection, Tennessee Department of Environment and Conservation, Florida Department of Health and Wisconsin Department of Health Services. Similarly, licenses for radioactive sources and materials are maintained at each of our international sites where such licenses are required, including in Belgium, China, Canada, Estonia, Finland, Germany, France, Japan and the Netherlands.
While the specific process and criteria for receiving a license differ from jurisdiction to jurisdiction, it generally involves an application process in which we: identify a person or persons who have appropriate training and experience to be a health physics/radiation safety officer; specify the radioactive sources or materials sought to be licensed, their physical form (i.e., sealed or unsealed) and maximum possession limits on the amount of each type of radioactive element or compound sought under the license; specify their intended use (e.g., calibration, testing, quality assurance, manufacturing); and, set forth written policies and procedures to ensure that we have adequate measures in place to ensure health and safety. These policies and procedures typically must be designed to ensure worker, workplace, and public safety, including emergency plans; set forth the proper handling, control and security of radioactive sources or materials on site; detail any disposal or decommissioning considerations; and adequately train personnel at the site in proper access to, and handling of, radioactive sources or materials.
The particular license requirements in a given jurisdiction are normally tailored to the specific radioactive elements or compounds involved, their physical form, and possession limits. Once authorities complete their application review and any required follow-up, the authority issues the site a license which imposes specific on-going compliance obligations that typically include requirements for us to pay periodic licensing fees, submit periodic written compliance reports, and agree to periodic site inspections by regulators, which may be announced or unannounced. Once a site has an existing license, the process for expanding or reducing the licensing scope generally is simpler than applying for a new license.
We have numerous licenses in effect at our various facilities in the United States, Canada, Finland, Germany, France, China, Japan, the Netherlands, Belgium and Estonia and the expiration dates of individual licenses differ by their term and effective date. Typical license terms range from two to five years, with authorities in some jurisdictions (e.g., Finland and Bavaria, Germany) issuing licenses that are perpetual subject to our on-going license compliance. For radioactive materials licenses in the United States, preapproval is generally required from the NRC or a U.S. State that has signed an agreement with the NRC authorizing such State to regulate certain radioactive materials within such State (an "Agreement State") before a direct or indirect transfer of a license, whether done through a sale or acquisition, restructuring, or other method. While specific regulations vary by jurisdiction, generally a license may be terminated by the regulatory authority immediately upon a finding of a substantial safety violation or other material violation of licensing requirements. For more minor violations, regulatory authorities typically provide the licensee with a written statement of deficiency or notice of violation stating required remediation steps, or requesting the licensee to identify corrective actions, and a demand for proof of remediation; depending on the severity of the violation, a re-inspection of the site may be performed by the authority to ensure adequate remedial steps have been completed.
In most cases, our various sites (including our predecessors) have held, maintained and (where required) renewed their licenses for a decade or more. In all cases, the licenses we require related to radioactive sources or materials are current and in force and, to the best of our knowledge, we are not aware of any basis to expect that any existing licenses subject to periodic renewals will not be renewed.
As a supplier of equipment and systems to the nuclear power industry, we are subject to regulations promulgated by the NRC that are applicable to vendors. Owners of nuclear power plants in the United States are licensed to build, operate, and maintain those plants by the NRC. Their license and applicable NRC regulations require that they qualify their suppliers and contractors to ensure that the suppliers and contractors comply with NRC regulations. The NRC has a robust inspection regime for commercial nuclear plants, which includes verification that, for example, design, procurement, maintenance, and radiation protection programs comply with NRC safety and quality assurance regulations and requirements.
Inspections of nuclear materials licensees are conducted frequently, in areas such as personnel training, radiation protection, and security of nuclear materials. Parts of the NRC’s inspection regime—including portions of 10 C.F.R. Part 21 on reporting of defects and noncompliance and Appendix B of 10 C.F.R. Part 50 related to Quality Assurance—are also directly applicable to contractors, suppliers, and other non-licensees. The NRC routinely conducts inspections at vendor sites on these matters and others. As a supplier to the nuclear power industry, we must demonstrate to our customers that we comply with NRC regulations related to quality assurance, reporting of defects and safety issues, security and control of personnel access and conduct. Section 170 of the AEA, which is also known as the Price-Anderson Act, supports the nuclear services industry by offering broad nuclear liability and insurance coverage and indemnification to commercial NPP operators and their suppliers, as well as Department of Energy, or DOE, contractors, for liabilities arising out of nuclear incidents at power plants licensed by the NRC and at DOE nuclear facilities. The indemnification authority of the NRC and DOE under the Price-Anderson Act was extended through 2025 by the Energy Policy Act of 2005. Our nuclear
power plant customers are covered by the nuclear liability insurance and indemnification provisions of the Price-Anderson Act. In addition, other jurisdictions have similar nuclear liability protection and indemnification regimes for nuclear facilities.
We deal with numerous U.S. and non-U.S. government agencies and entities, including the U.S. military, the armed forces of many NATO countries, the U.S. Department of Defense, the U.S. Department of State, the U.S. Department of Treasury, the NRC, the U.S. Department of Energy, the U.S. Department of Homeland Security and the corresponding governmental agencies and entities in the European Union and Canada. When working with these and other government agencies and entities, we must comply with, and are affected by, laws and regulations relating to the formation, administration and performance of contracts. These laws and regulations, among other things require certification and disclosure of all cost or pricing data in connection with various contract negotiations; impose acquisition regulations that define allowable and unallowable costs and otherwise govern our right to reimbursement under various cost-based U.S. government contracts; and restrict the use and dissemination of information classified for national security purposes and the exportation of certain products and technical data.
Export Controls
Our products and technologies are subject to export controls under the laws of the United States, Canada, the United Kingdom and the member states of the European Union. Depending on a number of factors, including the specific product or technology, the origin of that product or technology, the destination, the end-user and the end-use, exports of our products and technologies may require export licenses, permits or other authorizations from government export control authorities. Whether we will be able to conclude proposed transactions involving products or technologies that are subject to those export licensing requirements will depend on the relevant government agency’s determination on whether the proposed transaction is consistent with the exporting country’s national security and foreign policy interests.
As examples of export control laws and regulations potentially applicable to our products and technologies, our products, when manufactured in or exported from the United States, are subject to export controls under the U.S. Department of Energy’s Part 810 regulations (10 C.F.R. Part 810) governing transfer of commercial nuclear technology and assistance, the U.S. Commerce Department’s Export Administration Regulations ("EAR"), the U.S. State Department’s International Traffic in Arms Regulations ("ITAR"), or the Nuclear Regulatory Commission ("NRC"), export licensing regulations in 10 C.F.R. Part 110 governing exports of nuclear materials and equipment. Canadian and EU export control regimes have separate, sometimes overlapping requirements, which must also be considered for a proper export compliance system.
We have implemented detailed export control compliance procedures, in the form of our Export Management and Control Program ("EMCP"), to identify those products, technologies and transactions for which export licenses, permits or other authorizations are required, and to assure that all transactions are handled in accordance with all applicable export control laws and regulations. Among other things, the Mirion EMCP includes (i) third party service provider screening of all parties against the various governments’ lists of prohibited, restricted and sanctioned parties; (ii) end-use reviews and certification procedures; (iii) monitoring regulatory announcements; and (iv) periodic reviews of applicable export control regulations in order to assure that the compliance procedures are up to date and properly maintained. See “Part I, Item 1A. Risk Factors—Legal and Regulatory Risks—Legal compliance with import and export controls, as well as with sanctions, in the United States and other countries, is complex, and compliance restrictions and expenses could materially and adversely impact our revenue and supply chain.”
Economic Sanctions
Various United States laws and regulations implemented by the U.S. Treasury Department’s Office of Foreign Assets Control ("OFAC"), impose economic sanctions on certain countries, business entities and individuals. Those OFAC economic sanctions regulations: (i) impose comprehensive commercial and financial embargoes on transactions directly and indirectly with Cuba, Iran, North Korea, Syria or the Crimean Region, and Russia or certain regions of Ukraine more recently, including any entity or person located in those jurisdictions; and (ii) include a substantial list of persons and entities that have been determined to be closely affiliated with the government of an embargoed country, engaged in or supporting international terrorism, trafficking in narcotics, engaged in activities related to the proliferation of weapons of mass destruction, or otherwise acting in a manner contrary to United States foreign policy interests. United States persons (i.e., United States citizens, permanent residents and companies) are generally prohibited from engaging in any transaction which involves any property or any interest in property in which an embargoed country, a person in an embargoed country or a person on the OFAC list of sanctioned parties has an interest. The prohibitions on engaging in transactions with Cuba and Iran also extend to foreign subsidiaries of United States companies. Moreover, no United States person may approve, ratify, participate in, or otherwise “facilitate” any offshore transaction between a foreign company and any country, entity or person that is sanctioned under the OFAC economic sanctions regulations. The Department of Commerce’s Bureau of
Industry and Security ("BIS"), keeps an Entity List and other sanctions-related lists that are separate from the OFAC requirements.
Violations of United States export control regulations or the OFAC economic sanctions regulations are punishable by criminal and civil fines, imprisonment, loss of export privileges, debarment from United States Government contracts and, in extreme cases, listing on the OFAC list of sanctioned parties. See “Part I, Item 1A. Risk Factors—Legal and Regulatory Risks—Legal compliance with import and export controls, as well as with sanctions, in the United States and other countries, is complex, and compliance restrictions and expenses could materially and adversely impact our revenue and supply chain.”
Anti-Corruption Laws
We are subject to anti-bribery and anti-corruption laws, including the U.S. Foreign Corrupt Practices Act (the "FCPA"), the United Kingdom Bribery Act (the "UKBA"), and anti-corruption laws enacted in various other countries which implement the Organization of Economic Cooperation and Development (the "OECD"), Convention on Combating Bribery of Foreign Officials in International Business. Those laws generally prohibit any person or company from making payments to any “foreign official” for the purpose of obtaining or retaining business or obtaining any other unfair or improper advantage.
In particular, the FCPA prohibits any publicly traded company, or issuer, and any domestic concern from paying or giving, or promising or offering to pay or give, any money or any other thing of value directly or indirectly to a foreign official for the purpose of obtaining or retaining any business or obtaining any other unfair advantage. An issuer or domestic concern may be liable for penalties for violation of the FCPA if it make a payment, or provides any other thing of value, to a third party, such as a distributor, sales representative or other third party with knowledge that some or all of that money or thing of value will be paid or given to a foreign official for an improper purpose. In addition, the FCPA imposes upon issuers obligations to maintain complete and accurate books and records of account and to establish internal accounting controls, in order to prevent the diversion of corporate funds to the payment of bribes and other improper payments, and to prevent the establishment of “off books” accounts that might be used to fund improper payments to foreign officials.
Violations of the FCPA are punishable by criminal and civil fines and imprisonment and disgorgement of revenues derived from improper conduct. Any investigation or proceeding involving allegations of improper payments under the FCPA could materially and adversely affect our business, results of operations, financial condition, standing with customers, particularly government customers, and/or our business reputation. See “Part I, Item 1A. Risk Factors—Legal and Regulatory Risks—We must comply with the FCPA and analogous non-U.S. anti-bribery and anti-corruption statutes including the UKBA. Our or our sales representatives’ or distributors’ failure to comply with such laws could subject us to, among other things, penalties and legal expenses that could harm our reputation and materially and adversely affect our business, results of operations and financial condition.”
Compliance Procedures
To address the compliance challenges presented by the foregoing laws and regulations, we have adopted and implemented compliance policies and detailed compliance procedures. Our commitment to compliance with anti-corruption laws and regulations is memorialized in the Mirion Code of Ethics and Conduct, which sets forth our overall compliance policies and informs all of our employees of their compliance responsibilities. Our export controls and economic sanctions compliance policies are set forth in our EMCP and implemented at each of our sites via local procedures. Our compliance programs are reinforced with (i) ethics and compliance training for all employees; (ii) due diligence reviews of all prospective distributors, sales representatives and other third party intermediaries; (iii) detailed anti-corruption compliance contractual covenants in third-party agreements; (iv) detailed recordkeeping procedures; and (v) auditing of third parties’ business practices as needed.
Medical Device Regulation
We are required to register for permits and/or licenses with, obtain approvals from and comply with operating standards of the U.S. Food and Drug Administration (the "FDA"), the NRC, the U.S. Department of Health and Human Services (the "HHS"), the European Medicines Agency (the "EMA"), the U.K. Medicines and Healthcare Products Regulatory Agency (the "MHRA"), and other foreign agencies, and accrediting bodies depending upon the type of operations we are conducting and the location of product distribution, manufacturing and sale.
Many of our products in the medical end market, for instance our nuclear medicine products for cardiology, oncology, endocrinology, diagnostic radiology and radiation therapy; imaging products in the form of positioning devices, ultrasound tables and MRI stretchers; and our energy measurement products, including radiation monitoring and measuring instruments, are classified as medical devices and are subject to restrictions under domestic and foreign laws, rules,
regulations, self-regulatory codes, circulars, and orders, including, but not limited to, the U.S. Food, Drug, and Cosmetic Act (the "FDCA"). We incur a number of costs associated with obtaining and maintaining the approval to market our products. Furthermore, the FDA conducts detailed inspections of and controls over our manufacturing, marketing, distribution, import and export, record keeping and storage and disposal practices, together with various post-marketing requirements.
Specifically, the FDCA requires these products, when sold in the United States, to be safe and effective for their intended uses and to comply with the regulations promulgated and enforced by the FDA. The FDA regulates the design, development, research, preclinical and clinical testing, introduction, manufacture, advertising, labeling, packaging, marketing, distribution, import and export, and record keeping for such products.
Medical devices can be marketed only for the indications for which they are cleared or approved. After a device has received 510(k) clearance (a pathway for the FDA to approve a new medical device for marketing) for a specific intended use, any change or modification that significantly affects its safety or effectiveness, such as a significant change in the design, materials, method of manufacture, or intended use, may require a new 510(k) clearance and payment of an FDA user fee.
Any medical devices we manufacture and distribute are subject to pervasive and continuing regulation by the FDA, state and certain other comparable foreign authorities. As a medical device manufacturer, our manufacturing facilities are subject to inspection on a routine basis by the FDA and other comparable foreign authorities, as well as audits by our notified body in the European Economic Area, or EEA, as described below. We are required to adhere to the Current Good Manufacturing Practices requirements, as set forth in the Quality Systems Regulation, which require manufacturers, including third-party manufacturers, to follow stringent design, testing, control, documentation and other quality assurance procedures during all phases of the design and manufacturing process.
We must also comply with post-market surveillance regulations, including adverse event reporting requirements, which require that we review and report to the FDA and other comparable foreign authorities any incident in which our products may have caused or contributed to a death or serious injury. Further, we are required to report any incident in which our product has malfunctioned if that malfunction would likely cause or contribute to a death or serious injury if it were to recur.
Labeling, advertising and promotional activities are subject to scrutiny by the FDA and other comparable foreign authorities and, in certain circumstances, by the Federal Trade Commission and other foreign counterparts. Medical devices approved or cleared by the FDA, foreign regulators, or our notified bodies may not be promoted for undocumented, unapproved or uncleared uses, otherwise known as “off-label” promotion. The FDA, other U.S. agencies and other comparable foreign authorities actively enforce the laws and regulations prohibiting the promotion of off-label uses.
The FDA can withdraw marketing authorization for a medical device product if compliance with regulatory standards is not maintained or if problems occur after the product reaches the market. Later discovery of previously unknown problems with a product may result in restrictions on the product or complete withdrawal of the product from the market. Because our operations include the manufacture and distribution of nuclear medical products, we are also subject to regulation by the NRC and the departments of health of each state in which we operate, which leaves us with a complex collection of requirements to navigate.
Market access, sales and marketing of medical devices in non-U.S. countries are subject to foreign regulatory requirements that vary widely from country to country. The time required to obtain approval for marketing a medical device in a foreign country could be longer or shorter than the time required by the FDA. Furthermore, the requirements are different in each country. For example in the EEA, a medical device must meet the Medical Devices Directive’s (the "MDD"), Essential Requirements or the Medical Devices Regulation’s (the "MDR"), General Safety and Performance Requirements, if certified from May 26, 2021. Before placing a medical device on the EEA market, the manufacturer must prepare a declaration of conformity, certifying that the device complies with the MDD/MDR, and must then affix the CE mark. The notified body typically audits and examines the device’s technical documentation, and the quality system for the manufacture, design and final inspection of the relevant device before issuing a CE certificate. Following the issuance of this CE certificate, manufacturers may prepare the declaration of conformity and affix the CE mark to the devices covered by this CE certificate. Similar requirements apply in the UK. For access to the UK market, manufacturers must obtain a UKCA Certificate and affix a UKCA mark to their medical devices. However, the CE mark will be accepted in the UK until July 1, 2023.
The standard by which conformity with applicable standards and directives is measured is dependent upon the type and class of the product, but normally involves a combination of self-assessment by the manufacturer and a third party assessment by a notified body. In the European Union, or EU, the third party assessment may consist of an audit of the manufacturer’s quality system (currently ISO 13485), provisions of the MDD and specific testing of the manufacturer’s
device. Further, the MDR came into effect in the European Union on May 26, 2021, which requires us to obtain certification against the MDR to include a CE mark on new products, or make significant changes to existing products.
We are subject to additional regulations in other foreign countries, including, but not limited to, the United Kingdom and the EU to sell our products. We intend that either we or our distributors will receive any necessary approvals or clearance prior to marketing our products in those international markets.
We are subject to various healthcare related laws regulating fraud and abuse, research and development, pricing and sales and marketing practices, and the privacy and security of health information. In particular, the U.S. Federal Anti-Kickback Statute prohibits persons from knowingly and willfully soliciting, offering, receiving, or providing remuneration (including any kickback or bribe), directly or indirectly, in exchange for or to induce either the referral of an individual, or the furnishing or arranging for a good or service, for which payment may be made in whole or in part under a federal healthcare program, such as Medicare or Medicaid. A person or entity does not need to have actual knowledge of the statute or specific intent to violate it in order to have committed a violation. Similar laws and regulations apply in many foreign countries.
The Health Insurance Portability and Accountability Act of 1996 ("HIPAA"), prohibits knowingly and willfully (1) executing, or attempting to execute, a scheme to defraud any healthcare benefit program, including private payors, or (2) falsifying, concealing, or covering up a material fact or making any materially false, fictitious, or fraudulent statement in connection with the delivery of or payment for healthcare benefits, items, or services. In addition, HIPAA, as amended by the Health Information Technology for Economic and Clinical Health Act of 2009, also restricts the use and disclosure of patient identifiable health information, mandates the adoption of standards relating to the privacy and security of patient identifiable health information, and requires the reporting of certain security breaches with respect to such information. Similar to the U.S. Federal Anti-Kickback Statute, a person or entity does not need to have actual knowledge of the healthcare fraud statute implemented under HIPAA or specific intent to violate it in order to have committed a violation. Similar laws and regulations apply in many foreign countries.
The False Claims Act imposes liability on any person or entity that, among other things, knowingly presents, or causes to be presented, a false or fraudulent claim for payment by a federal healthcare program, knowingly makes, uses, or causes to be made or used, a false record or statement material to a false or fraudulent claim, or knowingly makes a false statement to avoid, decrease, or conceal an obligation to pay money to the U.S. federal government. The qui tam provisions of the False Claims Act allow a private individual to bring actions on behalf of the federal government alleging that the defendant has submitted a false claim to the federal government, and to share in any monetary recovery. In addition, the government may assert that a claim including items and services resulting from a violation of the U.S. Federal Anti-Kickback Statute constitutes a false or fraudulent claim for purposes of the civil False Claims Act. Similar laws and regulations apply in many foreign countries.
Federal consumer protection and unfair competition laws broadly regulate marketplace activities and activities that potentially harm consumers. Analogous U.S. state laws and regulations, such as state anti-kickback and false claims laws, also may apply to our business practices, including but not limited to, research, distribution, sales and marketing arrangements, and claims involving healthcare items or services reimbursed by any third-party payor, including private insurers. Further, there are state laws that require medical device manufacturers to comply with the voluntary compliance guidelines and the relevant compliance guidance promulgated by the U.S. federal government, or otherwise restrict payments that may be made to healthcare providers and other potential referral sources; state laws and regulations that require manufacturers to file reports relating to pricing and marketing information, which requires tracking gifts and other remuneration and items of value provided to healthcare professionals and entities; state and local laws requiring the registration of sales representatives; and state laws governing the privacy and security of health information in certain circumstances, many of which differ from each other in significant ways and often are not preempted by HIPAA. Similar laws and regulations apply in many non-U.S. countries.
Privacy and Information Security Laws
In the ordinary course of our business, we collect, store, use transmit and otherwise process certain types of data, including personal information, which subjects us to certain privacy and information security laws in the United States and internationally, including, for example and depending on the particular activity, the EU General Data Protection Regulation ("GDPR") and the California Consumer Privacy Act of 2018 ("CCPA"), and other laws, rules and regulations designed to regulate the processing of personal information and for example reduce risks of identity theft. These laws impose obligations with respect to the collection, processing, storage, disposal, use, transfer, retention and disclosure of personal information. In addition, under certain of these laws, we must provide notice to individuals of our policies and practices for sharing personal information with third parties, provide advance notice of any changes to our policies and in some cases give individuals the right to prevent processing of their personal information and disclosure of it to third parties. Further, all 50 states in the United States have laws including obligations to provide notification of unauthorized acquisition of
personal information to affected individuals, state officers and others. Some laws may also impose physical and electronic security requirements regarding the safeguarding of personal information. In order to comply with privacy and information security laws, we have confidentiality and information security standards and procedures in place for our business activities. Privacy and information security laws evolve regularly, and complying with these various laws, rules, regulations and standards, and with any new laws or regulations or changes to existing laws, could cause us to incur substantial costs that are likely to increase over time, requiring us to adjust our compliance program on an ongoing basis and presenting compliance challenges, change our business practices in a manner adverse to our business, divert resources from other initiatives and projects, and restrict the way products and services involving data are offered. See “Part I, Item 1A. Risk Factors—Legal and Regulatory Risks—Any actual or perceived failure to comply with evolving data privacy and data security laws and regulations in the jurisdictions where we operate, both inside and outside of the United States, could lead to government enforcement actions (which could include civil or criminal penalties), private litigation or adverse publicity and could materially and adversely affect our business.”
Available Information
Our website is www.mirion.com. The information found on, or that can be accessed from or that is hyperlinked to, our website is not part of this Annual Report on Form 10-K. We file or furnish annual, quarterly and current reports, proxy statements and other information with the United States Securities and Exchange Commission (“SEC”). You may obtain a copy of any of these reports, free of charge, from the Investors Relations section of our website as soon as reasonably practicable after we electronically file such material with, or furnish it to, the SEC. The SEC maintains an Internet site that also contains these reports at: www.sec.gov.
ITEM 1A. RISK FACTORS
An investment in our securities involves a high degree of risk. You should carefully consider the following risk factors, together with all of the other information included in this Annual Report on Form 10-K, before making an investment decision. The occurrence of one or more of the events or circumstances described in these risk factors, alone or in combination with other events or circumstances may have an adverse effect on our business, results of operations and financial condition. You should also carefully consider the following risk factors in addition to the other information contained in this Annual Report on form 10-K, including Part II, Item 7 “Management’s Discussion and Analysis of Financial Condition and Results of Operations” and the “Notes to Consolidated Financial Statements” of Part II, Item 8 “Financial Statements and Supplementary Data.” However, the selected risks described below are not the only risks we face. Additional risks and uncertainties not currently known to us or those we currently view to be immaterial may also materially and adversely affect our business, results of operations or financial condition. In such a case, the trading price of our securities could decline and you may lose all or part of your investment.
Summary of Principal Risk Factors
Below is a summary of some of the risks that we face. This summary is not complete, and should be read together with the entire section titled “Part I, Item 1A. Risk Factors” in this Annual Report on Form 10-K, as well as the other information in this Annual Report on Form 10-K and the other filings that we make with the SEC.
•Our global operations expose us to risks associated with public health crises and epidemics/pandemics, such as COVID-19. The global spread of COVID-19 has created significant volatility, uncertainty and worldwide economic disruption, resulting in an economic slowdown of potentially extended duration.
•We have incurred operating losses in the past and expect to incur operating losses in the future.
•Our results of operations may fluctuate significantly, which could make our future results difficult to predict and could cause our results of operations to fall below expectations.
•If we are unable to develop new products or enhance existing products to meet our customers’ needs and compete favorably in the market, we may be unable to attract or retain customers.
•We operate in highly competitive markets and in some cases compete against larger companies with greater financial resources.
•Our customers may reduce or halt their spending on our products and services.
•Our sales cycles in certain end markets can be long and unpredictable.
•Our growth plans depend in part on growth through acquisitions, and these plans involve numerous risks. If we are unable to make acquisitions, or if we are not successful in integrating the technologies, operations and personnel of acquired businesses or fail to realize the anticipated benefits of an acquisition, our business, results of operations and financial condition may be materially and adversely affected.
•Certain of our products require the use of radioactive sources or incorporate radioactive materials, which subject us and our customers to regulations, related costs and delays and potential liabilities for injuries or violation of environmental, health and safety laws.
•Accidents involving nuclear power facilities, including but not limited to events similar to Fukushima, or terrorist acts or other high profile events involving radioactive materials could materially and adversely affect our customers and the markets in which we operate and increase regulatory requirements and costs that could in turn materially and adversely affect our business.
•We have, and we intend to continue pursuing, fixed-price contracts. Our failure to mitigate certain risks associated with such contracts, such as inflation, may result in reduced margins.
•A failure to expand our manufacturing capacity if required, and scale our capabilities to manufacture new products could constrain our ability to grow our business.
•We rely on third-party manufacturers to produce sub-components for certain of our products and services. If our manufacturers are unable to meet our requirements, or are subject to unanticipated disruptions, our business, results of operations and financial condition could be materially and adversely affected.
•We rely on third-party sales representatives to assist in selling our products and services, and the failure of these representatives to perform as expected or to secure regulatory approvals in jurisdictions where they are required to do so could reduce our future sales.
•If we or our suppliers experience supply shortages, such as the ongoing shortage of semiconductors, or prices of commodities or components that we use in our operations increase, our results of operations could be materially and adversely affected.
•We derive a material portion of our revenue from contracts with governmental customers or their contractors and such customers may be subject to increased pressures to reduce expenses, require unusual or more onerous contractual terms and conditions or require that we undergo audits and investigations with an increased risk of sanctions and penalties.
•A failure or breach of our or our vendors’ information technology, or IT, data security infrastructure, or the security infrastructure of our products, or the discovery or exploitation of defects or vulnerabilities in the same, has subjected us in the past and may in the future subject us and our products to increased vulnerability to unauthorized access and other forms of cyberattacks and could materially and adversely impact our or our customers’ business, reputation, results of operations and financial condition.
•We and our customers operate in highly regulated industries that require us and them to obtain, and comply with, federal, state, local and foreign government permits and approvals.
•We operate in a highly litigious industry and adverse outcomes in any litigation may materially harm our business.
•We must comply with the FCPA, and analogous non-U.S. anti-bribery and anti-corruption laws statutes, including the UKBA. The failure by us or our third-party sales representatives’ or distributors’ to comply with such laws could subject us to, among other things, penalties and legal expenses that could harm our reputation and materially and adversely affect our business, results of operations and financial condition.
•Legal compliance with import and export controls, as well as with sanctions laws and regulations, in the United States and other countries, is complex, and compliance restrictions and expenses could materially and adversely impact our business, results of operations and financial condition.
•Certain of our products and software are subject to ongoing regulatory oversight by the FDA or equivalent regulatory agencies in international markets and if we are not able to obtain or maintain the necessary regulatory approvals we may not be able to continue to market and sell such products which may materially and adversely affect our business, results of operations and financial condition.
•Our ability to compete successfully and achieve future growth will depend on our ability to obtain, maintain, protect, defend and enforce our intellectual property and to operate without infringing, misappropriating or otherwise violating the intellectual property of others.
•The price of our Class A common stock and warrants may be volatile.
Risks Related to Our Business and Industry
Our global operations expose us to risks associated with public health crises and epidemics/pandemics, such as COVID-19. The global spread of COVID-19 has created significant volatility, uncertainty and worldwide economic disruption, resulting in an economic slowdown of potentially extended duration.
COVID-19 has had and may continue to have an adverse impact on our operations and supply chains, including as a result of impacts associated with preventive and precautionary measures that we, other businesses and governments are taking. More recently, the Delta and Omicron variants of the virus have contributed to a surge in COVID-19 cases globally and the full impact of the newly emerged Omicron variant has yet to be determined. The United States and other governments have reacted with an array of disparate laws and regulations, some of which have been challenged, which makes implementation and enforcement difficult and creates uncertainties for our and other businesses. Due to these impacts and measures, we have experienced unpredictable reductions in demand for certain of our products and services. Many employers in the United States and Europe, including us, are continuing to require some of their employees to work from home or not go into their offices or customers’ facilities. In addition to existing travel restrictions, countries may continue to close or decline to reopen borders, impose prolonged quarantines, and further restrict travel, which significantly impacts our ability to support our sites and customers in those locations and the ability of our employees to get to their places of work to produce products, or significantly hampers our products from moving through the supply chain. As a result, COVID-19 may materially adversely affect revenue growth in certain of our businesses, primarily those serving our medical end markets, and it is uncertain how materially COVID-19 will affect our global operations generally if these impacts were to persist or worsen over an extended period of time. The extent and duration of the impacts are uncertain and dependent in part on customers returning to work and economic activity ramping up.
The impact of COVID-19 on our customers has adversely affected our sales operations in certain ways. For example, we have experienced increased customer disputes regarding orders, delayed customer notices to proceed with production, delayed payment from customers and, on rare occasions, customers have refused to pay for their orders entirely.
Our ability to continue to manufacture products is highly dependent on our ability to retain, continue to hire and maintain the safety and health of our factory employees. COVID-19 has had and may continue to have an adverse impact on employees’ willingness to work onsite in our offices, including as a result of vaccine mandates in the United States and other countries, and we have experienced COVID-19 related attrition and have not been able to backfill certain employee roles or hire for open positions at certain sites. In addition, the ability of employees to work may be impacted by contracting or being exposed to COVID-19. While we are following the requirements of governmental authorities and taking preventative and protective measures to prioritize the safety of our employees, these measures may not be successful, and we may be required to temporarily close facilities or take other measures. For example, many of our facilities have undergone brief closures and/or severe limitations of onsite activities due to the COVID-19 pandemic. While we are staying in close communication with our sites, employees, customers and suppliers and acting to mitigate the impact of this dynamic and evolving situation, the duration and extent of the effect of COVID-19 on us is not determinable.
The duration and extent of the impact from the COVID-19 pandemic depends on future developments that cannot be accurately predicted at this time, such as the severity and transmission rate of the virus, the existence of any additional waves of the pandemic, the extent and effectiveness of containment actions, treatment and prevention measures, including vaccines, and the impact of these and other factors on our customers, employees, suppliers and other business partners. Moreover, to the extent the COVID-19 pandemic or any worsening of the global business and economic environment as a result thereof, continues to adversely affect our business and financial results, it may also have the effect of heightening or exacerbating many of the other risks described under “—Risks Related to Our Business Operations.”
We have incurred operating losses in the past and expect to incur operating losses in the future.
As of December 31, 2021, we had an accumulated deficit of $131.6 million. For the Successor Period from October 20, 2021 through December 31, 2021, the Predecessor Stub Period from July 1, 2021 through October 19, 2021, and fiscal
2021, we experienced net losses of $23.0 million, $105.7 million, $158.4 million, respectively. We cannot assure you that we will achieve positive net income in any future period. We expect our operating expenses to increase in the future as we expand our operations. Furthermore, as a public company, we are incurring additional legal, accounting and other expenses that we did not incur as a private company. If our revenue and gross profit do not grow at a greater rate than our operating expenses, we will not be able to achieve and maintain profitability. We expect to incur significant losses in the future for a number of reasons, including without limitation the other risks and uncertainties described herein. Additionally, we may encounter unforeseen operating or legal expenses, difficulties, complications, delays and other factors that may result in losses in future periods. If our expenses exceed our revenue, we may never achieve or maintain profitability and our business may be harmed.
Our results of operations may fluctuate significantly, which could make our future results difficult to predict and could cause our results of operations to fall below expectations.
Our business depends on the demand for our radiation detection, measurement, analysis and monitoring products, our nuclear medicine and related quality management products, and services in the nuclear, defense, medical and other end markets. In the past, the demand for our products in these markets has fluctuated due to a variety of factors, many of which are beyond our control. This has caused our results of operations to fluctuate. Among the factors affecting our results of operations are:
•general economic conditions, both domestically and internationally, including inflation, recession and interest rate fluctuations;
•the timing, number and size of orders from, and shipments to, our customers, as well as the relative mix of those orders;
•the timing of revenue recognition, which often requires customer acceptance of the delivered products;
•delays, postponements or cancellations of construction or decommissioning of NPPs caused by, for example, financing difficulties or regulatory delays;
•NPP outages, which are typically higher in the spring and fall due to reduced electricity demands
•adverse economic, financial and/or political conditions, as well as manmade or natural disasters, such as pandemics, in one or more of our target end markets;
•variations in the volume of orders for a particular product or product line in a particular quarter;
•the size and timing of new contract awards;
•the timing of the release of government funds for procurement of our products;
•the degree to which new end markets emerge for our products;
•seasonal customer purchasing patterns due to the budget cycles of U.S. and foreign governments and commercial enterprises that affect timing of order placement for or delivery of our products;
•the tendency of commercial enterprises to fully utilize annual capital budgets prior to expiration;
•international trade conditions, such as the tariffs imposed by both the United States and China on the import of certain goods; and
•changes in laws or regulations affecting our target end markets, in particular the medical market.
In addition, our operating results may be difficult to compare with our results for prior periods due to our recent change in fiscal year end from June 30 to December 31. As a result of these and other factors, you should not rely on the results of any prior quarterly or annual periods, or any historical trends reflected in such results, as indications of our future revenue or operating performance.
If we are unable to develop new products or enhance existing products to meet our customers’ needs and compete favorably in the market, we may be unable to attract or retain customers
The markets in which we compete are subject to technological changes, product obsolescence and evolving industry standards. Our ability to successfully compete in these markets and to continue to grow our business depends in significant part upon our ability to develop, introduce and sell new and enhanced products in a timely and cost-effective manner, and to anticipate and respond to changing customer requirements. We have experienced, and may in the future experience, delays in the development and introduction of new products.
These delays could provide a competitor a first-to-market advantage or greater market share. Defects or errors found in our products after commencement of commercial shipment could result in delays in market acceptance of these products. Our nuclear medicine and imaging products may become obsolete or unmarketable if new technologies are introduced to the market, or if new industry standards emerge. We may not be able to leverage our assets to diversify our products and services fast enough to generate revenue beyond our current markets in a timely manner. If we are unable to diversify our product and service offerings quickly enough to respond to market changes, our financial viability may worsen.
Our ability to successfully develop and introduce new products and product enhancements, and the revenues and costs associated with these efforts, will be affected by our ability to:
•properly identify and address customer needs;
•in the case of our medical end market, educate medical providers about the use of new products and services;
•comply with internal quality assurance systems and processes in a timely and efficient manner;
•manage regulatory approvals and clearances including their timing and costs;
•accurately predict and control costs associated with inventory overruns caused by phase-in of new products and phase-out of old products;
•manufacture and deliver our products in sufficient volumes on time and accurately predict and control costs associated with manufacturing, installation, warranty and maintenance of the products;
•meet our product development plan and launch timelines;
•improve manufacturing yields of components; and
•manage customer demands for retrofits of both old and new products.
Lack of market acceptance for our new products will jeopardize our ability to recoup research and development expenditures, hurt our reputation and harm our business, results of operations and financial condition.
Accordingly, we cannot assure you that our future product development efforts will be successful.
We operate in highly competitive markets and in some cases compete against larger companies with greater financial resources.
The market for our products and services is fragmented, with a variety of small and large competitors, where the degree of fragmentation and the identities of our competitors vary among our target end markets. Some of our competitors have greater financial resources than do we, and they may be able to focus those resources on developing products or services that are more attractive to potential customers than those that we offer, or on lobbying efforts to enhance their prospects of obtaining government contracts. Some of our competitors, for example, are substantially larger and better capitalized than we are and have the ability to combine solutions into an integrated offering at attractive prices. Our competitors may offer these solutions at prices below cost in order to improve their competitive positions. Any of these competitive factors could make it more difficult for us to attract and retain customers, cause us to lower our prices to compete, and reduce our market share and revenue, any of which could materially and adversely affect our business, results of operations and financial condition.
Supply shortages and continuing cost increases could materially and adversely affect our business, results of operations and financial condition.
During 2020 and 2021, we experienced a significantly stressed supply of labor, materials and freight, and we expect this to continue. The costs of materials and components of our products and the costs of labor and freight have been rising. In particular, some of our products incorporate microchips and other semiconductor components for which there is a global supply shortage. We also continue to operate in a labor constrained market and cannot predict future inflationary pressures or increases in tariffs on imported materials. Any inability to pass on future increased costs to customers would put downward pressure on our operating margins and materially and adversely affect our business, results of operations and financial condition.
Our customers may reduce or halt their spending on our products and services.
A variety of factors may cause our existing or future customers to reduce or halt their spending on our products and services. These factors include:
•unfavorable financial conditions and strategies of our customers;
•for the nuclear end market, civic opposition to or changes in government policies regarding nuclear operations or a reduction in demand for nuclear generating capacity;
•accidents, terrorism, natural disasters or other incidents occurring at our facilities, the facilities of our customers or at any other place; and
•the decision by one or more of our customers to acquire one of our competitors or otherwise insource the services we provide.
Our sales cycles in certain end markets can be long and unpredictable.
Our sales efforts for many of our products involve substantial discussion with customers regarding product configuration and deployment. This process can be extremely lengthy and time consuming and typically involves a significant product evaluation process. For example, the typical sales cycle for products whose procurement relates to the construction of new, or the refurbishment of existing, NPPs ranges from 12 to 36 months and has, in some cases, extended up to 60 months or more. In the medical end market, the typical sales cycle depends upon the type of product and whether the sales are international or within the United States, and can range from 1 to 18 months. In addition, these customers generally make a significant commitment of resources to test and evaluate our products prior to purchase. As a result, our sales process is often subject to delays associated with the lengthy approval processes that typically accompany the design, testing and adoption of new, technologically complex products. This results in us investing significant resources prior to orders being placed for our products, with no assurances that we will secure a sale.
In addition, a significant amount of time can pass before we recognize the revenue associated with an order once it has been placed. We may need a notice to proceed with an order from the customer before starting to execute the customer’s order, which may delay revenue recognition. We may also not recognize revenue for sales of certain of our products until the customer certifies the successful installation and operation of the product, which can be many months or, particularly with regard to our Sensing Systems and Radiation Monitoring Systems products, years following the receipt of a customer order. The installation of our equipment may also be subject to construction or scheduled outage delays unrelated to our products, which can further defer the recognition of revenue.
We exercise judgment in determining the timing of revenue by analyzing the point in time or the period over which the customer has the ability to direct the use of and obtain substantially all of the remaining benefits of the performance obligation. Revenue recognized on an over-time basis for the Predecessor Stub Period from July 1, 2021 to October 19, 2021 and the Successor Period from October 20, 2021 to December 31, 2021 accounted for approximately 26% and 22%, respectively, of total net sales. Typically, overtime revenue recognition is based on the utilization of an input measure used to measure progress, such as costs incurred to date relative to total estimated costs. Changes in total estimated costs are recognized using the cumulative catch-up method of accounting which recognizes the cumulative effect of the changes on current and prior periods in the current period. Accordingly, the effect of the changes on future periods of contract performance is recognized as if the revised estimate had been the original estimate. A significant change in an estimate on one or more contracts could have a material effect on our consolidated financial position, results from operations, or cash flows.
Our long and uncertain sales cycle and the unpredictable period of time between the placement of an order and our ability to recognize the revenue associated with the order makes revenue predictions difficult, particularly on a quarterly basis, and can cause our operating results to fluctuate significantly.
Our acquisition plans involve numerous risks. If we are unable to make acquisitions, or if we are not successful in integrating the technologies, operations and personnel of acquired businesses or fail to realize the anticipated benefits of an acquisition, our operations may be materially and adversely affected.
As part of our business and growth strategy, we have made and plan to continue to make acquisitions of, or significant investments, in businesses, products or technologies that allow us to complement our existing product offerings, expand our market coverage, increase our engineering workforce, reinforce our supply chain or enhance our technological capabilities.
For example, in fiscal 2020, we acquired Premium Analyse, a key player in the radioactive gas detection market and measurement of tritium, Selmic, an electronic component manufacturer of sensors, modules, and devices serving in automotive, transportation, medical, security, defense, and telecom industries, and Capintec, a leading supplier of calibration and measurement technologies for nuclear medicine applications. We also acquired the Personal Radiation Dosimeter facility from the Helmholtz Zentrum of Munich. From June 30, 2020 through the end of 2021 we acquired Biodex, a provider of nuclear instruments, imaging equipment and rehabilitation systems, DOSImetrics, a provider of personnel dosimetry systems, Sun Nuclear, a provider of radiation oncology quality assurance, and CIRS, a provider of medical imaging and radiation therapy phantoms, as well as a number of smaller dosimetry services businesses. We plan to continue exploring additional acquisition opportunities in the future but we are unable to predict whether or when any prospective acquisition candidate will become available or the likelihood that any acquisition will be completed. If our expected returns on these transactions are not achieved, it could adversely impact our business, results of operations and financial condition.
Even if we do find suitable acquisition opportunities, we may not be able to consummate the acquisitions on commercially acceptable terms or realize the anticipated benefits of any acquisitions we do undertake. Our ability to grow our business through acquisitions is subject to numerous risks, including competition for the acquisition of attractive or promising businesses or assets, the need to finance such acquisitions through cash on hand or debt or equity financing, and the need to secure required governmental approvals under antitrust and competition laws in the United States and worldwide. The sale of equity or equity-linked securities or issuance of debt to finance any such acquisitions could result in dilution to our stockholders. The incurrence of indebtedness would result in increased fixed obligations and could also include covenants or other restrictions that would impede our ability to manage our operations.
Where we succeed in acquiring a business or assets, we are exposed to many risks, including:
•problems integrating the new personnel or the purchased operations, technologies or products;
•difficulty securing adequate working capital;
•unanticipated costs associated with the acquisition;
•negative effects on our ability to generate excess free cash flow;
•negative effects on profitability;
•adverse effects on existing business relationships with suppliers and customers;
•risks associated with entering markets in which we have no or limited prior experience;
•loss of key employees of the acquired business;
•our assumption of legal or regulatory risks, particularly with respect to smaller businesses that have immature business processes and compliance programs;
•litigation arising from the operations before they were acquired by us; and
•difficulty completing financial statements and audits.
Our inability to overcome problems encountered in connection with any acquisition could divert the attention of management, consume scarce corporate resources and otherwise harm our business. If our expected returns on these transactions are not achieved, it could adversely impact our business, results of operations and financial condition.
Many of our products and services involve the detection, identification, measurement or monitoring of radiation and the failure of our products or services to perform to specification could materially and adversely affect our business, results of operations and financial condition.
Our products and services involve the detection and monitoring of radiation and are crucial components of the safety measures employed with respect to ionizing radiation. In the medical end market, our products and services are often used, for example, to ensure that radiation oncology patients receive accurate doses of radiation. In order to ensure the safety of such patients, we are committed to upholding high standards of precision and accuracy for our products. The failure of our products to perform to specification could result in personal injury or death and property damage (including environmental contamination), or the incorrect treatment being administered to patients. Legal and regulatory actions taken in response to product failure could result in significant costs to us. Additionally, the failure of our products to perform to specification could adversely affect market perception of the quality and effectiveness of our products and services, which would harm our ability to attract new customers and could cause our existing customers to cease doing business with us.
While we have attempted to secure appropriate insurance coverage at a reasonable cost, we do not insure against all risks and a claim can exceed the limits of our policies. We cannot assure you that our insurers will pay a particular claim, or that we will be able to maintain coverage at reasonable rates in the future, or at all. We may also be subject to significant deductibles.
Our contracts with customers generally seek to limit our liability in connection with product failure, but we cannot assure you that these contractual limitations on liability will be effective or sufficient in scope in all cases or that our insurance will cover the liabilities we have assumed under these contracts. The costs of defending against a claim arising out of such failure, and any damages awarded as a result of such a claim, could adversely affect our business, results of operations and financial condition.
Certain of our products require the use of radioactive sources or incorporate radioactive materials, which subject us and our customers to regulations, related costs and delays and potential liabilities for injuries or violation of environmental, health and safety laws.
The majority of our products designed to detect, quantify and analyze ionizing radiation require the use of radioactive sources for testing and calibration. The required radioactive sources, or other sources of ionizing radiation, e.g., X-ray machines, are held by our facilities performing these tests and calibrations. Our customers hold equivalent sources for ongoing testing and re-calibration. Customers often acquire the radioactive sources directly from third party providers but may also purchase the sources from us as accessory to the product.
Certain of our reactor instrumentation and control equipment and systems in our Industrial segment incorporate radioactive materials. In all such cases, licenses for radioactive sources and materials or other sources of ionizing radiation are provided by the appropriate regulatory authority in the relevant jurisdiction and such authorities may be at the state or national level. Our failure or any customer’s failure to obtain the necessary license for radioactive sources or materials required by or incorporated into our products could result in the cancellation or delay of purchases by our customers, or remedial action by the relevant regulators.
While the specific process and criteria for receiving a license differ from jurisdiction to jurisdiction, it generally involves policies and procedures designed to ensure worker, workplace and public safety, including emergency plans; setting forth the proper handling, control and security of radioactive sources or materials on site; detailing any disposal or decommissioning considerations; and adequately training personnel at the site in proper access to, and handling of, radioactive sources or materials.
Our noncompliance with, or failure to properly implement, such policies and procedures could delay or otherwise preclude us from obtaining the necessary license for radioactive sources or materials required by or incorporated into our products, which could result in the cancellation or delay of purchases by our customers. See “Part I, Item 1. Business—Regulation” for more information.
The particular license requirements in a given jurisdiction are normally tailored to the specific radioactive elements or compounds involved, their physical form and possession limits. Once authorities complete their application review and any required follow-up, the authority issues the site a license which imposes specific on-going compliance obligations that typically include requirements for us to pay periodic licensing fees, submit periodic written compliance reports, and agree
to periodic site inspections by regulators, which may be announced or unannounced. Our failure to comply with any of these on-going obligations could result in the revocation of the necessary license for radioactive sources or materials required by or incorporated into our products, which could result in the cancellation or delay of purchases by our customers.
We are subject to federal, state and local regulations governing storage, handling and disposal of these radioactive materials and waste products. Outside of the United States, we are also subject to radiation regulations that vary from country to country. The improper storage, use and disposal of such materials by us and/or our customers could result in direct or secondary liability, including penalties and fines, to us in the event of environmental contamination or physical injury. We cannot eliminate the risk of accidental contamination or injury from those radioactive materials nor can we control the practices of our customers. The sale and use of our products with radioactive sources or materials could also lead to the filing of claims if someone were to allege injury from the use of one of our products or allege that one of our products was defective. Such a claim could result in substantial damages, be costly and time-consuming to defend and adversely affect the marketability of our products and our reputation.
We and many of our customers operate in a politically sensitive environment, and the public perception of nuclear energy or nuclear medicine can affect our customers and us.
We and our customers operate in a politically sensitive environment. The risks associated with radioactive materials and the public perception of those risks can affect our business. Opposition by third parties can delay or prevent the construction of new nuclear power plants and can limit the operation of nuclear reactors. Adverse public reaction to developments in the use of nuclear power could directly affect our customers and indirectly affect our business. In the past, adverse public reaction, increased regulatory scrutiny and litigation have contributed to extended construction periods for new nuclear reactors, sometimes delaying construction schedules by decades or more or even shutting down operations. In addition, anti-nuclear groups in Germany successfully lobbied for the adoption of the Nuclear Exit Law in 2002, which requires the shutdown of all German NPPs by 2022. Adverse public reaction could also lead to increased regulation or limitations on the activities of our customers, more onerous operating requirements or other conditions that could have a material adverse impact on our customers and our business.
Accidents involving nuclear power facilities, including but not limited to events similar to Fukushima, or terrorist acts or other high profile events involving radioactive materials could materially and adversely affect our customers and the markets in which we operate and increase regulatory requirements and costs that could in turn materially and adversely affect our business.
Successful execution of our business model in the nuclear power end market is dependent upon a certain level of public support for nuclear power. Nuclear power faces strong opposition from certain competitive energy sources, individuals, and organizations. The accident that occurred at the Fukushima nuclear power plant in Japan beginning on March 11, 2011 increased public opposition to nuclear power in some countries, resulting in a slowdown in, or, in some cases, a complete halt to new construction of nuclear power plants, an early shut down of existing power plants, or a dampening of the favorable regulatory climate needed to introduce new nuclear technologies. As a result of the Fukushima accident, some countries that were considering launching new domestic nuclear power programs have delayed or cancelled the preparatory activities they were planning to undertake as part of such programs. If accidents similar to the Fukushima disaster or other events, such as terrorist attacks involving nuclear facilities, occur, public opposition to nuclear power may increase, regulatory requirements and costs could become more onerous and customer demand for our products in the nuclear end market could suffer, which could materially and adversely affect our business, results of operations and financial condition.
We enter into fixed-price contracts with our customers and our failure to mitigate certain risks associated with such contracts may result in reduced operating margins.
We estimate that approximately a quarter of our revenue was associated with contracts with a duration of 12 months or longer and approximately 60% of such revenue was associated with contracts with fixed-price arrangements which do not provide for price escalation in the event of unanticipated cost overruns, in each case for the fiscal year ended June 30, 2021. Under these contracts, we perform our services and provide our products at a fixed price. Fixed-price contracts carry inherent risks, including risks of losses from underestimating costs, operational difficulties and other changes that may occur over the contract period. We have in the past experienced unanticipated cost overruns on some of our fixed-price contracts. If our cost estimates for a contract are inaccurate or if we do not execute the contract within our cost estimates, we may incur losses or the contract may not be as profitable as we expected. In addition, even though some of our longer-term contracts contain price escalation provisions, such provisions may not fully provide for cost increases, whether from inflation, the cost of goods and services to be delivered under such contracts or otherwise. In addition, we are sometimes required to incur costs in connection with modifications to a contract that may not be approved by the customer as to scope or price, or to incur unanticipated costs, including costs for customer-caused delays, errors in specifications or designs or
contract termination, that we may not be able to recover. These, in turn, could materially and adversely affect our business, results of operations and financial condition.
The revenue, cost and gross profit realized on such contracts can vary, sometimes substantially, from the original projections due to changes in a variety of factors, such as:
•failure to properly estimate, or changes in, the costs of material, components or labor;
•inflation and currency exchange rate fluctuations;
•unanticipated technical problems with the products or services being supplied by us, which may require that we spend our own money to remedy the problem;
•our suppliers’ or subcontractors’ failure to perform;
•difficulties of our customers in obtaining required governmental permits or approvals;
•changes in local laws and regulations;
•unanticipated delays in construction of new NPPs and decommissioning of existing NPPs; and
•limited history with new products and new customers.
Furthermore, we intend to continue pursuing longer-term contracts which may continue to contain fixed-price arrangements, and the amount of revenue associated with such contracts may change in future periods. As a result of one or more of these factors, we may incur losses or contracts may not be as profitable as we expect, and this could materially and adversely affect our business, results of operations and financial condition.
We may not realize all of the sales expected from our backlog of orders and contracts, and amounts included in our order backlog may not result in actual revenue or translate into profits.
Although the amount of our backlog is based on signed purchase orders or other written contractual commitments, we cannot guarantee that our order backlog will result in actual revenue in the originally anticipated period or at all. As of December 31, 2021 and June 30, 2021 our estimated combined order backlog was $747.5 million and $715.8 million, respectively. The majority of our combined backlog is considered firm and expected to be delivered within two years. In addition, the mix of contracts included in our order backlog can greatly affect our margins in future periods, which may not be comparable to our historical product mix and operating results. Our customers may experience project or funding delays or cancel orders due to factors beyond our control. If customers terminate, reduce or defer firm orders, whether due to fluctuations in their business needs or purchasing budgets or other reasons, our sales will be adversely affected and we may not realize the revenue we expect to generate from our backlog or, if realized, the revenue may not translate into profit. We estimate approximately 10%-15% of our backlog at any point in time is related to contracts that are unfunded and may be at risk for cancellation if funding is not appropriated. If our order backlog fails to result in revenue in a timely manner or at all, we could experience an overall reduction in revenue and liquidity.
Risks Related to Our Business Operations
We operate as an entrepreneurial, decentralized company, which presents both benefits and certain risks. In particular, significant growth in a decentralized operating model may put strain on certain business group resources and our corporate functions, which could materially and adversely affect our business, results of operations and financial condition.
The business is organized in two reportable business segments: Medical and Industrial. Our Medical segment is based around our sales, products and services to customers in the medical market. The Industrial segment is primarily based around the nuclear energy, defense, laboratories and scientific research markets as well as other industrial markets.
The decentralization of our organization structure necessarily places significant control and decision-making powers in the hands of local management, which presents certain risks, including the risk that we may be slower to detect or react to compliance-related matters, that “company-wide” business initiatives may be more challenging or costly to implement, and the risk of noncompliance or failures is higher than they may be in a more centralized operating environment. In addition, key business group resources and our corporate functions, which are leanly staffed but responsible for supporting our decentralized operations, may also not be able to detect or resolve financial, operational, and compliance matters on a
timely basis. Our failure to adapt our financial, operational and compliance controls and systems to effectively manage our decentralized business and comply with our obligations as a public company could materially and adversely affect our business, results of operations and financial condition.
A failure to expand our manufacturing capacity if required, and scale our capabilities to manufacture new products could constrain our ability to grow our business.
While we currently have sufficient capacity, the future growth of our business may depend on our ability to successfully expand our manufacturing capacity. For example, we experienced manufacturing delays with one of our suppliers, Selmic, in connection with ramping up production of our Mirion Battlefield Dosimeter. To ensure on-time deliveries going forward, we acquired Selmic and invested resources in resolving the manufacturing issues that caused delays. Expansion of our manufacturing capacity may also require us to obtain regulatory approvals or additional financing. Delay in the expansion of our manufacturing capacity could constrain our ability to grow our business, which would materially and adversely affect our business, results of operations and financial condition.
Similarly, we could have substantial difficulty in dealing with rapid growth in markets for new products that we may introduce. If demand for our new products increases rapidly, we will need to expand internal production capacity or implement additional outsourcing. Success in developing, manufacturing and supporting products manufactured in small volumes does not guarantee comparable success in operations conducted on a larger scale. Manufacturing yields and product quality may decline as production volumes increase. If we are unable to deliver products quickly and cost effectively and in the requisite volumes, our customers may decline to purchase our new products or may purchase substitute products offered by our competitors. The costs associated with implementing new manufacturing technologies, methods, and processes, including the purchase of new equipment, and any resulting delays, inefficiencies and loss of sales, could harm our results of operations.
We rely on third-party manufacturers to produce sub-components for certain of our products and services. If our manufacturers are unable to meet our requirements, or are subject to unanticipated disruptions, our business could be harmed.
We use third-party manufacturers to produce sub-components for certain of our products. From time to time demand for our products has grown faster than the supply capabilities of these vendors. For example, significant growth in our Instadose product line required additional inventory purchasing to meet demand. In many cases, these manufacturers have no obligation to supply products to us for any specific period, in any specific quantity or at any specific price, except as set forth in a particular purchase order. Our requirements represent a small portion of the total production capacities for many of our manufacturers, and our manufacturers may reallocate capacity to other customers, even during periods of high demand for our products or services. We have in the past experienced, and may in the future experience, quality control issues and delivery delays with our manufacturers due to factors such as materials shortages, outages of specialized manufacturing equipment, high industry demand, inability of our manufacturers to consistently meet our quality or delivery requirements, or long lead times for components that could delay deliveries. Component manufacturers that sell to our suppliers may decide to stop producing certain components, declaring end-of-life for critical components and limiting supply of these components. In such cases, we would need to identify component alternatives, redesign electronic components or requalify electronic designs, which would require time and resources. In addition, third-party manufacturers may have financial difficulties and face the risk of bankruptcy, especially in light of the current worldwide economic downturn. If one of our suppliers was to cancel or materially change a commitment with us or fail to meet the quality or delivery requirements needed to satisfy customer orders for our products, we could lose time-sensitive customer orders, be unable to develop or sell our products or services cost effectively or on a timely basis, if at all, and have significantly decreased revenue, which would harm our business, results of operations and financial condition. We may qualify additional suppliers in the future which would require time and resources. If we do not qualify additional suppliers, we may be exposed to increased risk of capacity shortages due to our dependence on our current suppliers.
In addition, our suppliers (and those they depend upon for materials and services) are subject to risks, including COVID-19-related supplier plant shutdowns or slowdowns, labor disputes or constraints, union organizing activities, intellectual property claims, financial liquidity, information technology failures, inclement weather, natural disasters, significant public health and safety events, supply constraints, and general economic and political conditions that could limit their ability to provide us with materials. Insurance for certain disruptions may not be available, affordable or adequate. The effects of climate change, including extreme epidemics and pandemics, weather events, long-term changes in temperature levels, sea level rise and water availability may exacerbate these risks. Such disruption has in the past and could in the future interrupt our ability to manufacture certain products.
We derive a significant portion of our revenue from international sales and our operations in foreign countries are subject to political, economic, legal and other risks, which could materially and adversely affect us.
Revenue generated from outside of North America accounted for approximately 40%, 36%, and 45% of our net sales for the Successor Period from October 20, 2021 through December 31, 2021, the Predecessor Stub Period from July 1, 2021 through October 19, 2021, and in fiscal 2021, respectively, and approximately 48% of our net sales in both fiscal 2020 and 2019. We anticipate that international sales will continue to constitute a material percentage of our total net sales in future periods. As a result, our operations are subject to risks associated with global operations and sales, including:
•foreign currency exchange fluctuations;
•changes in regulatory requirements;
•tariffs and other barriers;
•timing and availability of export licenses;
•difficulties in accounts receivable collections;
•difficulties in protecting and enforcing our intellectual property;
•difficulties in staffing and managing international operations;
•difficulties in managing sales agents, distributors and other third parties;
•coordination regarding, and difficulties in obtaining, governmental approvals for products that may require certification;
•rescission or termination of contracts by governmental parties without penalty and regardless of the terms of the contract;
•restrictions on transfers of funds and other assets of our subsidiaries between jurisdictions;
•the burden of complying with a wide variety of complex foreign laws and treaties;
•potentially adverse tax consequences; and
•uncertainties relative to regional political and economic circumstances.
We are also subject to risks associated with the imposition of legislation and regulations relating to the import or export of our products. Furthermore, the failure to comply with export control regulations and to obtain required approvals could result in loss of the ability to continue to export products, fines and penalties.
We cannot predict whether quotas, duties, taxes or other charges or restrictions upon the importation or exportation of our products will be implemented by the United States or other countries. Some of our customers’ purchase orders and agreements are governed by foreign laws, which often differ significantly from the laws of the United States. Therefore, we may be limited in our ability to enforce our rights under such agreements and to collect damages, if awarded. These factors may materially and adversely affect our business, results of operations and financial condition.
We rely on third-party sales representatives to assist in selling our products and services, and the failure of these representatives to perform as expected or to secure regulatory approvals in jurisdictions where they are required to do so could reduce our future sales.
We derive a significant portion of our revenue from sales through third-party sales representatives. We have established relationships with some of our third-party sales representatives recently, and we are unable to predict the extent to which our third-party sales representatives will be successful in marketing and selling our products and services. Moreover, many of our third-party sales representatives also market and sell competing products and services, which may affect the extent to which our third-party sales representatives promote our products and services. If our third party sales representatives advertise or promote or characterize our products in a manner inconsistent with our (or their) messaging, as approved by our regulatory affairs professionals, such acts could be imputed to us and we could become subject to risk or liability from government regulatory bodies or agencies for criminal or civil claims, including false claims, and we could become susceptible to individual consumer actions or class actions based on false or improper advertising and promotion, off-label promotion, failure to warn defects in our products and unfair competition or unfair trade practices claims, all of which
could lead to adverse publicity, fines, penalties, judgments, money damages and other significant losses. Our future performance will also depend, in part, on our ability to attract additional third-party sales representatives who will be able to market and support our products and services effectively and accurately, especially in markets in which we have not previously sold our products and services. If we cannot retain our current third-party sales representatives or recruit additional or replacement third-party sales representatives, our business, results of operations and financial condition could be harmed.
If our suppliers experience supply shortages and prices of commodities or components that we use in our operations increase, our results of operations could be materially and adversely affected.
We are dependent upon certain sole or limited source suppliers for critical raw materials or components of some of our products. For example, we rely on limited source suppliers for certain precious metals used in some of our radiation oncology and reactor instrumentation, scintillator materials used in our detection and identification equipment, analog sensor tubes used in certain of our imaging products, and detectors used in our dosimetry line of products.
Most of our suppliers are not required to supply us with any minimum quantities, and we cannot assure you that we will receive adequate quantities of components on a timely basis in the future. For example, a single source supplier informed us that its supplier was discontinuing the manufacture of an on-board computer module component of one of our multi-channel analyzers used by our Industrial segment to interpret signals from our detectors allowing our customers to understand levels of detectable radiation. The notification prompted us to secure a final end-of-life order in an amount sufficient to meet our anticipated production requirements at least through April 2022, the exact duration depending on sales of this particular device. Qualification and redesign efforts are underway to meet this timeline.
Our suppliers could have financial or other issues that could cause a disruption in the supply or increase the cost of components to us. Such disruptions or delays could impact our obligations to other parties. In addition, were we to change suppliers of components in some of our products, we may be required to seek new qualifications for such products, which can be a time-consuming and costly process. As a result of interruption of supply or increased component costs, we may not be able to obtain the raw materials or components that we need to fill customer orders. The inability to fill these orders could cause delays, disruptions or reductions in product shipments, require us to negotiate alternate supply arrangements with replacement suppliers where available or require product redesigns which could, in turn, damage relationships with current or prospective customers, increase costs or prices and materially and adversely affect our business, results of operations and financial condition, including through litigation.
Our reliance upon sole or limited sources of supply for certain materials or components could cause production interruptions, delays and inefficiencies.
We purchase materials, components, and equipment from third parties for use in our manufacturing operations. For example, we purchase cryogenic cooling equipment to support our spectroscopy line of products. There is a limited supply market for this type of equipment, and these products are designed specifically for use in our products. Qualification and design of new equipment will require time and resources to complete. Our income could be adversely impacted if we are unable to adjust our purchases to reflect changes in customer demand and market fluctuations, including those caused by seasonality or cyclicality. During a market upturn, suppliers may extend lead times, limit supplies, or increase prices. If we cannot purchase sufficient products at competitive prices and quality and on a timely enough basis to meet increasing demand, we may not be able to satisfy market demand, product shipments may be delayed, our costs may increase, or we may breach our contractual commitments and incur liabilities. Conversely, in order to secure supplies for the production of products, we sometimes enter into non-cancelable purchase commitments with vendors, which could impact our ability to adjust our inventory to reflect declining market demands. If demand for our products is less than we expect, we may experience additional excess and obsolete inventories and be forced to incur additional charges and our profitability may suffer.
In addition, some of our businesses purchase certain requirements from sole or limited source suppliers for reasons of quality assurance, cost effectiveness, availability, contractual obligations or uniqueness of design or technology. If these or other suppliers encounter financial, operating, quality, or other issues or if our relationship with them changes, including as a result of contractual disputes, we might not be able to quickly establish or qualify replacement sources of supply. The supply chains for our businesses could also be disrupted by supplier capacity constraints, operational or quality issues, bankruptcy or exiting of the business for other reasons, decreased availability of key raw materials or commodities, and external events such as natural disasters, pandemic health issues, war, terrorist actions, governmental actions, and legislative or regulatory changes. Any of these factors could result in production interruptions, delays, extended lead times, and inefficiencies. As discussed above, such disruptions could also result in liability from litigation.
Because we cannot always immediately adapt our production capacity and related cost structures to changing market conditions, our manufacturing capacity may at times exceed or fall short of our production requirements. Any or all of these issues could result in the loss of customers, provide an opportunity for competing products to gain market acceptance, and otherwise adversely affect our profitability. If we are not able to mitigate the impact of any disruptions in our supply chain, then our business, results of operations and financial condition may be materially and adversely impacted.
Because we compete directly with certain of our customers and suppliers, our results of operations could be materially and adversely affected in the short term if these customers or suppliers abruptly discontinue or significantly modify their relationship with us.
Some of our competitors are also our suppliers and customers. For example, we had an arrangement with a supplier of components used to manufacture our Cryo-Cycle product. That supplier was acquired by one of our competitors, after which time the supplier ceased supplying us with the components used to manufacture the Cryo-Cycle. As with our other suppliers, our competitor suppliers are not required to supply us with any minimum quantities, and we cannot assure you that we will receive adequate quantities of components on a timely basis in the future. The loss of orders stemming from the actions of our supplier or customer competitors could cause delays, disruptions or reductions in product shipments or require product redesigns that could, in turn, damage relationships with current or prospective customers, increase costs or prices, result in litigation or otherwise materially and adversely affect our business, results of operations and financial condition.
We derive a material portion of our revenue from contracts with governmental customers or their contractors, and such customers may be subject to increased pressures to reduce expenses, require unusual or more onerous contractual terms and conditions or require that we undergo audits and investigations with an increased risk of sanctions and penalties.
U.S. government contractors and subcontractors must comply with specific procurement regulations and other requirements, including without limitation those related to ethics and business conduct, cost accounting, pricing, intellectual property, employment, cybersecurity, and supply chain issues. Accordingly, we are subject to routine audits and investigations by U.S. government agencies and held to strict compliance standards. If we fail to comply and demonstrate our compliance with these rules and regulations, we could be subject to reductions in the value of our government contracts, contract modification or termination, loss of valuable intellectual property rights, the assessment of criminal and civil penalties and fines, and/or suspension or debarment from government contracting and subcontracting for a period of time or permanently.
Furthermore, we have bid, and may in the future submit bids, for U.S. government contracts that require our employees to maintain various levels of security clearances and require us or our subsidiaries to maintain certain facility security clearances in compliance with Department of Defense or Department of Energy requirements. Obtaining and maintaining security clearances for employees involves a lengthy process, and it can be difficult to identify, recruit and retain employees who already hold security clearances. If our employees are unable to obtain or retain security clearances, or if our employees who hold security clearances stop working for us, we may face delays in fulfilling contracts, or be unable to fulfill or secure new contracts, with any customer involved in classified work. Any breach of security for which we are responsible could seriously harm our business, damage our reputation and make us ineligible to work on any classified programs.
The classified work that we currently perform at one of our facilities subjects us to the industrial security regulations of the Department of Defense that are designed to safeguard against unauthorized access by foreigners and others to classified and other sensitive information. We may be subject to penalties for violations of these regulations and the U.S. government could terminate our contracts with it or decide not to renew them and such a situation could also impair our ability to obtain new contracts and subcontracts. The government may also change its procurement practices or adopt new contracting rules and regulations that could be costly to satisfy or that could impair our ability to obtain new contracts. See “Legal and Regulatory Risks—We must comply with the U.S. Foreign Corrupt Practices Act and analogous non-U.S. anti-bribery and anti-corruption statutes including the UK Anti-Bribery Act. Our third-party sales representatives’ or distributors’ failure to comply with such laws could subject us to, among other things, penalties and legal expenses that could harm our reputation and materially and adversely affect our business, results of operations and financial condition.”
Any reduction in the capital resources or government funding of our customers could reduce our sales and impede our ability to generate revenue.
A significant portion of our sales are capital purchases by our customers. The spending policies of our customers could have a significant effect on the demand for our products. These policies are based on a wide variety of factors, including the resources available to make purchases, the spending priorities among various types of equipment, policies regarding spending during recessionary periods and changes in the political climate. In particular, certain customers can come under significant budgetary pressure and resort to cost-cutting measures.
Any changes in capital spending or changes in the capital budgets of our customers could significantly reduce demand for our products. The capital resources of our customers may be limited by the availability of equity or debt financing. In addition, a portion of our sales are to governmental and non-profit entities such as universities and hospitals, which are subject to unique budgetary pressures. Any reduction in spending or budget austerity measures could inhibit the ability of these customers to purchase our products.
Many of our large contracts have penalties for late deliveries.
In some cases, including through many of our fixed-price contracts, we have agreed to deliver a project by a scheduled date. If we fail to deliver the project as scheduled, we may be held responsible for costs associated with the delay, generally in the form of liquidated damages, in some cases up to the full value of the contract. We have in the past incurred penalties associated with late delivery on some of our contracts. In the event that a project is delayed, the total costs of the project could exceed our original estimates, and we could experience reduced profits or a loss for that project.
A failure or breach of our or our vendors’ information technology (“IT”) data security infrastructure, or the security infrastructure of our products, or the discovery or exploitation of defects or vulnerabilities in the same, has subjected us in the past and may in the future subject us and our products to increased vulnerability to unauthorized access and other forms of cyberattacks and could materially and adversely impact our or our customers’ business, reputation, results of operations and financial condition.
We rely upon the capacity, reliability and security of our and our vendors’ IT and data security infrastructure and our and our vendor’s ability to expand and continually update this infrastructure in response to the changing needs of our business. As we implement new systems or integrate existing systems, they may not perform as expected, which may result in liability or incurred costs, including litigation. We also face the challenge of supporting our older systems and implementing necessary upgrades. If we experience an issue with the functioning of an important IT system or a security breach of our IT systems, including during system upgrades and/or new system implementations, the resulting disruptions, including because of investigations or litigation, could have a material and adverse effect on our business, results of operations and financial condition. We are indirectly exposed to the same risks in our supply chain. Furthermore, we collect and maintain information in digital form that is necessary to conduct our business, and we are increasingly dependent on our IT and data security infrastructure to operate our business. In the ordinary course of our business, we collect, store and transmit large amounts of confidential information, including intellectual property, proprietary business information and personal information. It is critical that we do so in a secure manner to maintain the confidentiality and integrity and privacy of such confidential information. We have established physical, electronic and organizational measures to safeguard and secure our systems to prevent data compromise and rely on commercially available systems, software, tools, and monitoring to provide security for our IT systems and the processing, transmission and storage of digital information. We have also outsourced elements of our IT systems and, as a result, a number of third-party vendors may or could have access to our confidential information.
Despite our implementation of security measures, our IT systems, like those of other companies, are vulnerable to damage or interruption from a variety of sources, including physical damage, telecommunications or network failures or interruptions, system malfunction, natural disasters, malicious human acts, terrorism and war. Such IT systems, including our servers, are additionally vulnerable to physical or electronic break-ins, security breaches from inadvertent or intentional actions by our employees, third-party service providers, contractors, consultants, business partners, and/or other third parties, or from cyber-attacks by malicious third parties (including the deployment of harmful malware, ransomware, denial-of-service attacks, social engineering, and other means to affect service reliability and threaten the confidentiality, integrity, and availability of information). For example, in February 2021, we experienced a ransomware attack that involved the unauthorized access to certain of our servers. While we were able to detect and stop the unauthorized access before any substantial amount of information was accessed and before the attacker was able to encrypt our systems, the attacker misappropriated certain personal and proprietary information and publicly published certain of such information. We reported the incident to the applicable government authorities in France, Germany and the United States. Additionally, one of our acquired subsidiaries experienced a ransomware attack in February 2020, prior to our acquisition of such subsidiary. The acquired subsidiary did not make any ransom payments and was able to restore its systems from backups. Although we have implemented additional security measures to prevent future ransomware attacks, we can provide no assurance that our IT systems, or those of the third parties upon which we rely, will not experience cybersecurity incidents in the future. The risk of a security breach or disruption, particularly through cyber-attacks or cyber intrusion, including by computer hackers, foreign governments, and cyber terrorists, has generally increased as the number, intensity, and sophistication of attempted attacks and intrusions from around the world have increased. We may not be able to anticipate all types of security threats, and we may not be able to implement preventive measures effective against all such security threats. The techniques used by cyber criminals change frequently, may not be recognized until launched, and can originate from a wide variety of sources, including outside groups such as external service providers, organized crime affiliates, terrorist organizations, or hostile foreign governments or agencies. It is possible that we or our third-party vendors may
experience cybersecurity and other breach incidents that remain undetected for an extended period. Even when a security breach is detected, the full extent of the breach may not be determined immediately. The costs to us to mitigate network security issues, bugs, viruses, worms, malicious software programs and security vulnerabilities could be significant and, while we have implemented security measures to protect our IT and data security infrastructure, our efforts to address these issues may not be successful. There is also the potential for class action or other litigation as the result of such issues and the dissemination of personal information.
Any system failure, accident or security breach could result in disruptions to our operations or those of our customers. A material network breach in the security of our IT systems could include the theft of our intellectual property (including our trade secrets), customer information, human resources information or other confidential matter or the theft of the confidential information of our customers. To the extent that any disruption or security breach results in a loss or damage to our or our customers’ data, or an inappropriate disclosure of confidential, proprietary or customer information, it could cause significant damage to our reputation, affect our relationships with our customers, lead to claims against us, including civil litigation, and ultimately harm our business. In addition, we may be required to incur significant costs to protect against damage caused by these disruptions or security breaches in the future. If our IT systems fail and our redundant systems or disaster recovery plans are not adequate to address such failures, or if our business interruption insurance does not sufficiently compensate us for any losses that we may incur, our revenues and profits could be reduced and the reputation of our brand and our business could be materially and adversely affected.
We are also reliant on the security practices of our third-party service providers, which may be outside of our direct control. The services provided by these third parties are subject to the same risk of outages, other failures and security breaches described above. If these third parties fail to adhere to adequate security practices, or experience a breach of their systems, the data of our employees, customers and business associates may be improperly accessed, used or disclosed. In addition, our providers have broad discretion to change and interpret the terms of service and other policies with respect to us, and those actions may be unfavorable to our business operations. Our providers may also take actions beyond our control that could harm our business, including discontinuing or limiting our access to one or more services, increasing pricing terms, terminating or seeking to terminate our contractual relationship altogether, or altering how we are able to process data in a way that is unfavorable or costly to us. Although we expect that we could obtain similar services from other third parties, if our arrangements with our current providers were terminated, we could experience interruptions in our business, as well as delays and additional expenses in arranging for alternative cloud infrastructure services. Any loss or interruption to our systems or the services provided by third parties would adversely affect our business, results of operations and financial condition.
Failure to secure and protect our trade secrets or other confidential or proprietary information from disclosure or misappropriation could materially and adversely affect our business, competitiveness, results of operations and financial condition.
We rely on trade secrets and confidentiality agreements to protect our unpatented know-how, technology, and other proprietary information, including unpatented proprietary radiation detection expertise, continuing technological innovation and other trade secrets some of which is licensed from third parties, and to develop and maintain our competitive position. With respect to our products, we consider trade secrets and know-how to be one of our primary sources of intellectual property rights. However, trade secrets and know-how can be difficult to protect. We seek to protect these trade secrets and other proprietary technology, in part, by entering into non-disclosure and confidentiality agreements with parties who have access to them, such as our employees, corporate collaborators, outside contractors, consultants, advisors, and other third parties. We also enter into confidentiality and invention or patent assignment agreements with our employees and consultants. We cannot guarantee that we have entered into such agreements with each party that may have or have had access to our trade secrets or proprietary information, including our technology and processes. Despite these efforts, any of these parties may breach the agreements and disclose our proprietary information, including our trade secrets, and we may not be able to obtain adequate remedies for such breaches. Enforcing a claim that a party illegally disclosed or misappropriated a trade secret is difficult, expensive, and time-consuming, and the outcome is unpredictable. In addition, some courts inside and outside the U.S. are less willing or unwilling to protect trade secrets. If any of our trade secrets were to be lawfully obtained or independently developed by a competitor or other third party, we would have no right to prevent them from using that technology or information to compete with us. If any of our trade secrets were to be disclosed to or independently developed by a competitor or other third party, it could materially and adversely affect our competitive position, business, results of operations and financial condition.
Our future success is dependent on our ability to retain key personnel, including our executive officers, and attract qualified personnel. If we lose the services of these individuals or are unable to attract new talent, our business will be materially and adversely affected.
Our future operating results depend in significant part upon the continued contributions of our key technical and senior management personnel, many of whom would be difficult to replace. We are particularly dependent on the continued service of Thomas D. Logan, our Chief Executive Officer and Brian Schopfer, our Chief Financial Officer.
Our future operating results also depend in significant part upon our ability to attract, train and retain qualified management, manufacturing and quality assurance, engineering, marketing, sales and support personnel. In particular, engineers skilled in the analog technologies used in certain of our products are in high demand and competition to attract such personnel is intense. In addition, the expected increase in construction of new NPPs may exacerbate the shortage of radiation engineers and other qualified personnel. We are continually recruiting such personnel; however, we cannot assure you that we will be successful in attracting, training or retaining such personnel now or in the future. There may be only a limited number of persons with the requisite skills to serve in these positions, and it may be increasingly difficult for us to hire such persons over time. The high demand for such personnel may increase the costs to us to recruit and retain employees.
The loss of any key employee, the failure of any key employee to perform in his or her current position, our inability to attract, train and retain skilled employees as needed or the inability of our officers and key employees to expand, train and manage our employee base could materially and adversely affect our business, results of operations and financial condition.
If we encounter manufacturing problems, or if our manufacturing facilities do not continue to meet federal, state or foreign manufacturing standards, we may be required to temporarily cease all or part of our manufacturing operations, which would result in delays and lost revenue.
Many of our products are complex and require the integration of a number of components from several sources of supply. We must manufacture and assemble these complex systems in commercial quantities in compliance with regulatory requirements and at an acceptable cost. In addition, the COVID-19 pandemic may impact the supply of key components such that we may not receive them in a timely manner, in sufficient quantities, or at reasonable cost. We may also experience limitations in the availability of qualified personnel as a result of shelter-in-place rules, quarantine requirements, or illness. If our manufacturing capacity does not keep pace with product demand, we will not be able to fulfill orders in a timely manner, which in turn may breach our obligations to our business partners or otherwise have a negative effect on our financial results and overall business, including as a result of litigation. Conversely, if demand for our products decreases, the fixed costs associated with excess manufacturing capacity may adversely affect our financial results.
Our manufacturing processes and the manufacturing processes of our third-party suppliers are required to comply with the FDA’s Quality System Regulations (“QSR”), which are medical device good manufacturing practices for any products imported into, or sold within, the United States. The QSR is a complex regulatory scheme that covers all aspects of medical device manufacture, from pre-production design validation and servicing, as such aspects bear upon the safe and effective use of the device and whether the device otherwise meets the U.S. Federal Food, Drug and Cosmetic Act (“FDCA”). Other jurisdictions where our medical device products are distributed and sold have their own regulatory requirements that include quality and manufacturing requirements and controls. Furthermore, we are required to verify that our suppliers maintain facilities, procedures and operations that comply with our quality requirements. We are also subject to state licensing and other requirements and licenses applicable to manufacturers of medical devices, and we are required to comply with International Organization for Standardization, or ISO, quality system standards in order to produce products for sale in Europe and Canada, as well as various other foreign laws and regulations. Because our manufacturing processes include the production of diagnostic and therapeutic X-ray equipment and laser equipment, we are subject to the electronic product radiation control provisions of the FDCA, which requires that we file reports with the FDA, applicable states and our customers regarding the distribution, manufacturing and installation of these types of equipment. The FDA enforces the QSR and the electronic product radiation control provisions through inspections, both periodic and for cause. We have been, and will continue being subject to such inspections. FDA inspections usually occur every two to three years. During such inspections, the FDA may issue Inspectional Observations on Form FDA 483, listing instances where a manufacturer has failed to comply with the FDCA, applicable regulations and procedures, or previous warning letters.
Sometimes inspections result in warning letters which are publicly available and can result in adverse publicity. Our failure to take prompt and satisfactory corrective action in response to an adverse inspection or our failure to comply with applicable regulatory requirements and standards could result in enforcement actions, including a shutdown of our manufacturing operations, a recall of our products, civil or criminal penalties, or other sanctions, which would cause our sales and business to suffer. Any inspection or government action based on alleged violations of law could require us to expend significant time and resources in response and could generate negative publicity. The occurrence of any event or penalty described above may inhibit our ability to keep our products on the market and generate revenue. In addition, because some foreign regulatory approvals require approvals or clearances from the FDA, any failure to comply with FDA requirements may also disrupt our sales of products in other countries. We cannot assure you that the FDA or other
governmental authorities would agree with our interpretation of applicable regulatory requirements, or that we, or our third-party suppliers, have in all instances fully complied with all applicable requirements. If any of these events occur, our reputation could be harmed, we could lose customers and our business, results of operations and financial condition could be materially and adversely affected, including as the result of litigation.
If we cannot achieve the required level and quality of production, we may need to outsource production or rely on licensing and other arrangements with third parties who possess sufficient manufacturing facilities and capabilities in compliance with regulatory requirements. Even if we could outsource needed production or enter into licensing or other third-party arrangements, this could reduce our gross margin and expose us to the risks inherent in relying on others. We also cannot assure you that our suppliers will deliver an adequate supply of required components on a timely basis, or that they will adequately comply with the QSR. Failure to obtain these components on a timely basis would disrupt our manufacturing processes and increase our costs, which would harm our operating results.
The localization requirements in certain of our markets, in particular in Russia, China, India and South Korea, could limit our ability to sell our products.
Many emerging markets, including Russia, China, India and South Korea, impose localization requirements sometimes as a condition to funding contracts, which favor locally based component manufacturers and which require some degree of technology transfer to local manufacturers. Over time, such localization requirements could limit our ability to sell into such markets and could affect our ability to maintain our trade secrets. If our ability to sell our products in these markets is restricted, our business, results of operations and financial condition could be materially and adversely affected.
Our operations, and the operations of our suppliers, distributors or customers, could be subject to natural and manmade disasters and other business disruptions, which could materially and adversely affect our business and increase our expenses.
Our operations could be subject to natural disasters and other business disruptions, which could lead to reductions of revenue and increases in costs and expenses. For example, some of our facilities are located in areas with earthquake fault lines or in hurricane zones. In the event of a major earthquake or other natural or manmade disaster, we could experience business interruptions, destruction of or damage to facilities and/or loss of life, any of which could materially and adversely affect our business, results of operations and financial condition.
Our management has limited experience in operating a public company. The requirements of being a public company may strain our resources and divert management’s attention, and the increases in legal, accounting and compliance expenses may be greater than we anticipate.
We are a public company, and as such we incur significant legal, accounting and other expenses that we did not incur as a private company. We are subject to the reporting requirements of the Exchange Act, and are required to comply with the applicable requirements of the Sarbanes-Oxley Act and the Dodd-Frank Act, as well as the rules and regulations subsequently implemented by the SEC and the listing standards of the NYSE, including changes in corporate governance practices and the establishment and maintenance of effective disclosure and financial controls.
Compliance with these rules and regulations can be burdensome. Our management and other personnel need to devote a substantial amount of time to these compliance initiatives. Moreover, these rules and regulations have increased, and will continue to increase, our historical legal and financial compliance costs and will make some activities more time-consuming and costly. For example, we expect that these rules and regulations may make it more difficult and more expensive for us to attract and retain qualified members of our board of directors as compared to a private company. In particular, we incur significant expenses and devote substantial management effort toward ensuring compliance with the requirements of Section 404 of the Sarbanes-Oxley Act. We have hired additional accounting and financial staff, and engaged outside consultants, all with appropriate public company experience and technical accounting knowledge and maintain an internal audit function, which increased our operating expenses.
Our executive officers have limited experience in the management of a publicly traded company. Their limited experience in dealing with the increasingly complex laws pertaining to public companies could be a significant disadvantage in that it is likely that an increasing amount of their time may be devoted to these activities, which will result in less time being devoted to the management and growth of the post-combination company. We may not have adequate personnel with the appropriate level of knowledge, experience and training in the accounting policies, practices or internal control over financial reporting required of public companies. Our management will need to continually assess our staffing and training procedures to improve our internal control over financial reporting. Further, the development, implementation, documentation and assessment of appropriate processes, in addition to the need to remediate any potential deficiencies, will require substantial time and attention from management. The development and implementation of the standards and controls necessary for us to achieve the level of accounting standards required of a public company may require costs
greater than expected. It is possible that we will be required to expand our employee base and hire additional employees to support our operations as a public company which will increase its operating costs in future periods.
As a private company, we were not required to document and test our internal controls over financial reporting, our management was not required to certify the effectiveness of our internal controls and our auditors were not required to opine on the effectiveness of our internal controls over financial reporting. Failure to maintain adequate financial, information technology and management processes and controls could result in material weaknesses which could lead to errors in our financial reporting, which could adversely affect our business.
We were not required to document and test our internal controls over financial reporting, our management was not required to certify the effectiveness of our internal controls and our auditors were not required to opine on the effectiveness of our internal controls over financial reporting. As a large accelerated filer we are now subject to Section 404 of the Sarbanes-Oxley Act. However, we are required to provide management’s attestation on internal controls commencing with our annual report for the year ending December 31, 2022, and our auditors will be required to opine on the effectiveness of internal controls for this period. We may not be able to complete our evaluation, testing and any required remediation in a timely fashion. In addition, our current controls and any new controls that we develop may become inadequate because of poor design and changes in our business, including increased complexity resulting from our international operations and our contemplated international expansion. Any failure to implement and maintain effective internal controls over financial reporting could adversely affect the results of assessments by our independent registered public accounting firm and their attestation reports.
If we are unable to certify the effectiveness of our internal controls, or if our internal controls have a material weakness, we may not detect errors timely, our financial statements could be misstated, we could be subject to regulatory scrutiny and a loss of confidence by stakeholders, which could harm our business and adversely affect the trading price of our Class A common stock.
We identified a material weakness in our internal control over financial reporting, and we may experience additional material weaknesses or otherwise fail to design and maintain effective internal control over financial reporting, our ability to timely and accurately report our results of operations and financial condition in compliance with reporting requirements applicable for public companies in the United States could be impaired, which may adversely affect investor confidence in us and, as a result, the value of our Class A common stock or our warrants.
As previously disclosed in the GSAH Annual Report on Form 10-K/A filed on May 17, 2021, GSAH identified a material weakness in internal control over financial reporting as of December 31, 2020. A material weakness is a deficiency, or a combination of deficiencies, in internal control over financial reporting, such that there is a reasonable possibility that a material misstatement of the Company’s annual or interim financial statements will not be prevented or detected on a timely basis. Specifically, we concluded that our control around the interpretation and accounting for certain complex features of the Class A common stock and warrants issued by us was not effectively designed or maintained. This material weakness resulted in the restatement of our financial statements as of and for the year ended December 31, 2020, our balance sheet as of July 2, 2020, and our interim financial statements for the quarter ended September 30, 2020. Additionally, this material weakness could result in a misstatement of the warrant liability, Class A common stock and related accounts and disclosures that would result in a material misstatement of the financial statements that would not be prevented or detected on a timely basis.
Subsequent to the Business Combination on October 20, 2021, and upon filing of this Annual Report on Form 10-K for the period ended December 31, 2021, the internal controls over financial reporting of Mirion Technologies, Inc. replaced the internal controls over financial reporting of GSAH. As a result, the internal control structure of GSAH is no longer in operation. Instead, the relevant internal control structure after completion of the Business Combination is that of Mirion Technologies, Inc.
During the Successor Period ended December 31, 2021, we implemented the below changes to our processes to improve our internal control over financial reporting to remediate the control deficiency that gave rise to the material weakness:
•While we have processes to properly identify and evaluate the appropriate accounting technical pronouncements and other literature for all significant or unusual transactions, we have enhanced these processes to ensure that the nuances of such transactions are effectively evaluated in the context of the increasingly complex accounting standards. We require the formalized consideration of obtaining additional technical guidance prior to concluding on all significant or unusual transactions.
•We acquired enhanced access to accounting literature, research materials and documents and increased communication among our personnel and third-party professionals with whom we consult regarding the application of temporary and permanent equity and complex accounting transactions.
After completion of the above changes, our management believes the previously identified material weakness has been remediated. See "Part II. Item 9A. Controls and Procedures."
Effective internal controls are necessary for us to provide reliable financial reports and prevent fraud. If we are unable to develop and maintain effective internal control over financial reporting we may not be able to accurately report our financial results in a timely manner, which may cause us to be unable to comply with securities law or applicable stock exchange requirements, adversely affect investor confidence in us and/or materially and adversely affect our business, results of operations and financial condition, and our stock price may decline as a result. Any required remediation measures may be time consuming and costly and there is no assurance that any measures taken to date or any such measures taken in the future will ultimately have the intended effects, including to avoid potential future material weaknesses.
Our reported financial results may be affected by changes in accounting principles generally accepted in the United States.
Generally accepted accounting principles in the United States (“GAAP” or “U.S. GAAP”) are subject to interpretation by the Financial Accounting Standards Board (“FASB”) the SEC and various bodies formed to promulgate and interpret appropriate accounting principles. A change in these principles or interpretations could have a significant effect on our reported financial results, and could affect the reporting of transactions completed before the announcement of a change. Any difficulties in implementing any future changes to accounting principles could cause us to fail to meet our financial reporting obligations, which could result in regulatory discipline and harm investors’ confidence in us.
Legal and Regulatory Risks
We are subject to, or may otherwise be impacted by, a variety of federal, state, local and foreign laws and regulatory regimes. Failure to comply with such laws and regulations could subject us to, among other things, penalties and legal expenses which could materially and adversely affect our business, results of operations and financial condition.
Our business is subject to regulation by various federal, state, local and foreign governmental agencies. In the United States, such regulation includes the radioactive material exposure and nuclear facilities regulatory activities of the NRC, the anti-trust regulatory activities of the Federal Trade Commission and Department of Justice, the import/export regulatory activities of the Department of Commerce, the Department of State and the Department of Treasury, the regulatory activities of the Occupational Safety and Health Administration, the regulations of the FDA, the environmental regulatory activities of the Environmental Protection Agency, the labor regulatory activities of the Equal Employment Opportunity Commission and tax and other regulations by a variety of regulatory authorities in each of the areas in which we conduct business. We are also subject to regulation in other countries where we conduct business. In certain jurisdictions, such regulatory requirements may be more stringent than in the United States. We are also subject to a variety of U.S. federal and state employment and labor laws and regulations, including the Americans with Disabilities Act, the Federal Fair Labor Standards Act, the Worker Adjustment and Restructuring Notification Act, which requires employers to give affected employees at least 60 days’ notice of a plant closing or mass layoff, and other regulations related to working conditions, wage-hour pay, overtime pay, employee benefits, anti-discrimination and termination of employment. We are also subject to the employment and labor laws and regulations of the foreign jurisdictions, including France and Germany, where many of our employees are located.
Noncompliance with applicable regulations or requirements could subject us to investigations, sanctions, enforcement actions, disgorgement of profits, fines, damages, civil and criminal penalties, injunctions or debarment from government contracting or subcontracting. In addition, from time to time we have received, and may in the future receive, correspondence from former employees terminated by us who threaten to bring claims against us alleging that we have violated one or more labor or employment regulations. An adverse outcome in any such litigation could require us to pay damages.
Governmental enforcement actions could harm our business, results of operations and financial condition. If any governmental sanctions are imposed, or if we do not prevail in any civil or criminal litigation, our business, results of operations and financial condition could be materially and adversely affected. In addition, responding to any action could be costly and result in a significant diversion of management’s attention and resources.
We and our customers operate in highly regulated industries that require us and them to obtain, and comply with, federal, state, local and foreign government permits and approvals.
We and our customers operate in a highly regulated environment. Many of our products and services must comply with various domestic and international standards that are used by regulatory and accreditation bodies for approving such
services and products. Many of our products, particularly those offered by our Industrial segment, are subject to an array of product testing under extreme temperature, pressure, radiation and seismic conditions, known collectively as a qualification, for any given nuclear reactor design. The qualification is typically owned by the party who pays for the testing and so, in certain cases, we license such qualifications from a third party. In addition, many of our products and services, particularly those offered by our Medical segment, must be certified by the National Voluntary Laboratory Accreditation Program in the United States and by other governmental agencies in international markets. The termination of any such accreditation or our failure to obtain and maintain required qualification or accreditation for our products and services may adversely affect our revenue and results of operations.
Changes in these standards and accreditation requirements may also result in our having to incur substantial costs to adapt our products. Such adaptations may introduce quality assurance issues following such adaptation as new features and products may not perform as expected. Additionally, changes affecting radiation protection practices, including new understandings of the hazards of radiation exposure and corresponding changes in regulations, may impact how our services are used by our customers and may, in some circumstances, cause us to alter our products and services.
Our subsidiary Sun Nuclear offers oncology quality assurance products for diagnostic imaging and radiation therapy. These products may be relied upon by customers as part of their quality assurance programs for regulatory compliance, and thus could subject the company to potential risk of regulatory noncompliance or enforcement action by state or federal regulatory agencies, including but not limited to the NRC, Agreement State radiation safety agencies, the FDA, the Center for Disease Control and Prevention (“CDC”) and other agencies.
In addition, our customers are required to obtain, and to comply with, federal, state, local and foreign government licenses, permits and approvals with respect to either their facilities or possession and use of radioactive sources or other radioactive materials. For example, federal agencies such as the NRC and FDA, Agreement State agencies, and others have certain regulatory responsibilities regarding medical devices, radiopharmaceuticals, and other medical products that utilize radioactive material. Any of these licenses, permits or approvals may be subject to denial, revocation or modification under various circumstances. Failure to obtain or comply with the conditions of licenses, permits or approvals may adversely affect our customers’ operations by suspending their activities or delaying or preventing the receipt of radioactive sources or other radioactive materials, and may subject them to penalties and other sanctions. Although existing licenses, permits or approvals are routinely renewed by various regulators, renewal could be denied or jeopardized by various factors, including but not limited to:
•failure to comply with environmental and safety laws and regulations;
•failure to comply with permit conditions or violations found during inspections or otherwise;
• local community, political or other opposition;
•executive action; and
•legislative action.
Furthermore, if new environmental legislation or regulations are enacted or existing laws or regulations are amended or are interpreted or enforced differently, our customers may be required to obtain additional operating licenses, permits or approvals. Regulatory issues experienced by our customers may lead to delay or cancellation of their orders for our products and services or the discontinuance of future orders. We cannot assure you that we or our customers will be able to meet all potential regulatory challenges.
Changes in industry standards and governmental regulations may increase our expenses or reduce demand for our products or services.
We compete in markets in which we and our customers must comply with supranational, federal, state, local, and other jurisdictional regulations, such as regulations governing health and safety, the environment, and electronic communications, and market standardizations. We develop, configure, and market our products and services to meet customer needs created by these regulations and standards. These regulations and standards are complex, change frequently, have tended to become more stringent over time, and may be inconsistent or conflicting across jurisdictions. Any significant change or delay in implementation in any of these regulations or standards (or in the interpretation, application, or enforcement thereof) could reduce or delay demand for our products and services, increase our costs of producing or delay the introduction of new or modified products and services, or could restrict our existing activities, products, and services. In addition, in certain of our markets our growth depends in part upon the introduction of new regulations or implementation of industry standards on the timeline we expect. In these markets, the delay or failure of
governmental and other entities to adopt or enforce new regulations or industry standards, or the adoption of new regulations or industry standards which our products and services are not positioned to address, could adversely affect demand. In addition, regulatory deadlines or industry standard implementation timelines may result in substantially different levels of demand for our products and services from period to period.
Changes in global or regional environmental conditions and governmental actions in response to climate changes may materially and adversely affect us.
There is growing concern from many members of the scientific community and the general public that an increase in global average temperatures due to emissions of greenhouse gases and other human activities have caused, and will continue to cause, significant changes in weather patterns and increases in the frequency and severity of natural disasters. Government mandates, standards or regulations intended to reduce greenhouse gas emissions or projected climate change impacts have resulted, and are likely to continue to result, in operational constraints and cause us to incur expenses that will place pressure on margins or that will require us to increase the price of our products and services to the point that it affects demand for those products and services.
We operate in a highly litigious industry and are subject to risks related to legal claims and proceedings filed by or against us, and adverse outcomes in these matters may materially harm our business.
We are subject to various claims, disputes, investigations, demands, arbitration, litigation, or other legal proceedings. Legal claims and proceedings may relate to labor and employment, commercial arrangements, intellectual property, disputes with customers or business partners, breach of contract, environmental, health and safety, property damage, theft, consumer protection, class action, mass tort and product liability, personal injury, false advertising, unfair competition or unfair trade practices, public or private nuisance, “whistleblower” litigation, fiduciary duties of our directors and officers, securities, Medicare and Medicaid reimbursement claims, false claims, radioactive contamination, indemnity, insurance and various other matters. Legal matters are inherently uncertain and we cannot predict the duration, scope, cost, outcome or consequences of such matters.
Legal matters are expensive and time-consuming to defend, settle, and/or resolve, even if successfully, and may require us to implement certain remedial measures that could prove costly or disruptive to our business and operations and could result in civil or criminal fines, penalties, consent decrees, changes in business practices and exclusion from participation in various government healthcare-related programs. The unfavorable resolution of one or more of these matters could have an adverse impact on our business, results of operations and financial condition.
We may incur material losses and expenses as a result of products liability claims brought against us.
We face an inherent business risk of exposure to products liability claims, with or without merit. This includes where our products are found to be defective in design or manufacture, a misstatement is found on product labels or marketing materials, including (but not limited to) in product warnings and instructions, or where our or our agents’ conduct is found to fall below the standard of care for a similarly situated medical device company.
Accordingly, we should expect, in the ordinary course of business, to encounter class actions, mass tort actions, claims that allege our marketed products or products in development are mislabeled, mischaracterized or defective and violate applicable consumer protection statutes or FDA regulations or have caused, or could cause, serious adverse events or injury, including latent injury, and claims that our products have been, or should be recalled due to safety or warning defects. As discussed above, if our insurance coverage is inadequate to cover such claims or actions, we must pay the amount of any settlement or judgment in excess of the policy limits. Our failure to maintain adequate insurance coverage or failure to successfully defend against such claims, lawsuits and issues could materially and adversely affect our business, results of operations and financial condition.
Legal, political and economic uncertainty surrounding the exit of the United Kingdom from the European Union and the implementation of the trade and cooperation agreement between the United Kingdom and the European Union could materially and adversely affect our business.
In June 2016, voters in the United Kingdom approved a referendum to withdraw the United Kingdom’s membership from the European Union, which is commonly referred to as “Brexit.” The United Kingdom’s withdrawal from the European Union occurred on January 31, 2020, but the United Kingdom remained in the European Union’s customs union and single market for a transition period that expired on December 31, 2020. On December 30, 2020, the United Kingdom and the European Union entered into the Trade and Cooperation Agreement, which was applied on a provisional basis from January 1, 2021. While the economic integration does not reach the level that existed during the time the United Kingdom was a member state of the European Union, the Trade and Cooperation Agreement sets out preferential arrangements in areas such as trade in goods and in services, digital trade and intellectual property. Negotiations between the United
Kingdom and the European Union are expected to continue in certain other areas which are not covered by the Trade and Cooperation Agreement. The long-term effects of Brexit will depend on the effects of the implementation and application of the Trade and Cooperation Agreement and any other relevant agreements between the United Kingdom and the European Union.
We have operations in the United Kingdom and the European Union and, as a result, we face risks associated with the potential uncertainty and disruptions that may follow Brexit and the implementation and application of the Trade and Cooperation Agreement, including with respect to volatility in exchange rates and interest rates, disruptions to the free movement of data, goods, services, people and capital between the United Kingdom and the European Union and potential material changes to the regulatory regime applicable to our operations in the United Kingdom. The uncertainty concerning the United Kingdom’s future legal, political and economic relationship with the European Union could adversely affect political, regulatory, economic or market conditions in the European Union, the United Kingdom and worldwide and could contribute to instability in global political institutions, regulatory agencies and financial markets. These developments, or the perception that any of them could occur, have had and may continue to materially and adversely affect global economic conditions and the stability of global financial markets and could significantly reduce global market liquidity and limit the ability of key market participants to operate in certain financial markets. In particular, it could also lead to a period of considerable uncertainty in relation to the United Kingdom financial and banking markets, as well as to the regulatory process in Europe. Asset valuations, currency exchange rates and credit ratings may also be subject to increased market volatility.
We may also face new regulatory costs and challenges as a result of Brexit that could materially and adversely affect our operations. For example, as of January 1, 2021, the United Kingdom lost the benefits of global trade agreements negotiated by the European Union on behalf of its members, which may result in increased trade barriers that could make our business activities in areas that are subject to such global trade agreements more difficult. In addition, Brexit could lead to legal uncertainty and potentially divergent national laws and regulations as the United Kingdom determines which laws of the European Union to replace or replicate. There may continue to be economic uncertainty surrounding the consequences of Brexit that adversely impact customer confidence resulting in customers reducing their spending budgets on our services, which could materially adversely affect our business, results of operations and financial condition.
The ongoing instability and uncertainty surrounding Brexit and the implementation and application of the Trade and Cooperation Agreement, could require us to restructure our business operations in the United Kingdom and the European Union and could have an adverse impact on our business and employees in the United Kingdom and European Union.
Enhanced international tariffs, including tariffs that affect our products or components within our products, other trade barriers or global trade wars or domestic preferences could increase our costs and materially and adversely affect our business, results of operations and financial condition.
Our global business could be negatively affected by trade barriers and other governmental protectionist measures, any of which can be imposed suddenly and unpredictably. There is currently significant uncertainty about the future trade relationships between the United States and various other countries, most significantly China, with respect to trade policies, treaties, government regulations and tariffs. Since the beginning of 2018, there have been increasing public threats and, in some cases, legislative or executive action, from United States and foreign leaders regarding instituting tariffs against foreign imports of certain materials. During the last half of calendar year 2018, the federal government imposed a series of tariffs ranging from 7.5% to 25% on a variety of imports from China (the “Section 301 Duties”). These tariffs affect certain components that we import into the United States from our suppliers. China has responded to these Section 301 Duties with retaliatory tariffs ranging from 5% to 25% on a wide range of products from the United States, which include certain of our products. Higher duties on existing tariffs and further rounds of tariffs have been announced or threatened by the United States and Chinese leaders. Although the United States and China signed an initial trade deal in January 2020 and China announced a one year tariff exemption for medical linear accelerators in September 2019, there is no assurance that the trade deal will be signed or that the exemption on medical linear accelerators will continue beyond one year or that we will continue to qualify for such exemption.
These tariffs are subject to a number of uncertainties as they are implemented, including future adjustments and changes. Section 301 Duties on certain categories of imported Chinese products have been challenged in a case currently pending before the United States Court of International Trade. The outcome of that judicial challenge to the Section 301 Duties is uncertain. Moreover, even if the Court of International Trade determines that the imposition of the Section 301 Duties on those Chinese products was unauthorized and outside the scope of the U.S. Trade Representative’s statutory authority under Section 301 of the Trade Act of 1974, it is uncertain how the Chinese Government will respond with respect to continued application of Chinese retaliatory duties on United States origin products.
In 2020 and 2021, the United States Government threatened to impose additional duties under section 301 of the Trade Act of 1974 on various categories of products imported from a number of countries, including particularly the United Kingdom
and certain European Union member states, in response to the imposition or proposed imposition by those countries of a digital services tax. That Section 301 proceeding was terminated by the United States Trade Representative in November, 2021, as the parties agreed to negotiate a comprehensive agreement on digital services taxation under the auspices of the Organization for Economic Cooperation and Development’s Inclusive Framework on Base Erosion and Profit Shifting. In the event that no agreement is reached, however, or if various countries proceed with the unilateral imposition of a digital services tax or its equivalent, the United States Trade Representative could restart those Section 301 proceedings, which could result in increased customs duties on products that we import into the United States from our suppliers and our affiliated companies in the United Kingdom and in European Union member states.
Additionally, the United States has threatened to impose tariffs on goods imported from other countries, which could also impact our or our customers’ operations. If these tariffs continue, if additional tariffs are placed on certain of our components or products, or if any related counter-measures are taken by China, the United States or other countries, our business, results of operations and financial condition may be materially harmed. The ultimate reaction of other countries and the impact of these tariffs or other actions on the United States, China, the global economy and our business, results of operations and financial condition, cannot be predicted at this time, nor can we predict the impact of any other developments with respect to global trade. The imposition of tariffs could also increase our costs and require us to raise prices on our products, which may negatively impact the demand for our products in the affected market. If we are not successful in offsetting the impact of any such tariffs, our revenue, gross margins and operating results may be adversely affected.
These developments may materially and adversely affect global economic conditions and the stability of global financial markets, and they may significantly reduce global trade and, in particular, trade between China and the United States. Any of these factors could depress economic activity, restrict our access to customers and materially and adversely affect our business, results of operations and financial condition.
We must comply with the U.S. Foreign Corrupt Practices Act and analogous non-U.S. anti-bribery and anti-corruption laws including the UK Bribery Act. Our third-party sales representatives’ or distributors’ failure to comply with such laws could subject us to, among other things, penalties and legal expenses that could harm our reputation and materially and adversely affect our business, results of operations and financial condition.
We are required to comply with the United States Foreign Corrupt Practices Act (“FCPA”) which makes it unlawful to engage in bribery or to make any payments or provide any other benefits, directly or indirectly, to foreign officials for the purpose of obtaining or retaining business or to secure any other improper advantage. The FCPA also requires us, as a publicly traded company, to keep accurate books, records and accounts, and to maintain an effective system of internal accounting controls.
We operate, directly or indirectly, in more than one hundred countries around the world, many of which pose a high risk of corruption. In many countries, we also have government customers, and we utilize a network of third-party sales representatives and distributors. Based on these factors and others, our business involves a significant risk of potential FCPA violations.
All Mirion employees are informed of our responsibilities under the FCPA in the Mirion Code of Ethics and Conduct, and compliance with the FCPA is specifically mandated in detailed provisions of our agreements with third-party sales representatives and distributors. In addition, we provide live training on FCPA compliance on a regular basis for our employees who are involved in functions that necessitate such knowledge and training. Before we became a public company, we were not subject to the accounting provisions of the FCPA. Nevertheless, as a matter of course, we continuously review and, when warranted, update and enhance our systems, procedures, contracting processes, third-party due diligence, auditing and recordkeeping to address our FCPA compliance obligations and mitigate FCPA compliance risk. In spite of this, based on the jurisdictions where we operate, the fact that we have government customers, and our use of a network of third-party sales representatives and distributors, there remains a risk that one or more employees or third parties, acting on behalf of Mirion, might engage in conduct for which we might be held responsible under the FCPA. On occasion, we may terminate distribution or other agreements with sales channel partners operating in certain non-U.S. jurisdictions based on our ongoing compliance program. This could materially impact our ability to do business in jurisdictions where we are unable to enter into agreements with alternative partners that meet our compliance standards, which could materially and adversely impact our competitive position in such jurisdictions, as well as our business, results of operations and financial condition.
If our employees, third-party sales representatives and distributors or other agents are found to have engaged in such practices, we could suffer (i) severe penalties, including criminal and civil penalties, disgorgement, temporary or permanent debarment from public contracts, and (ii) other remedial measures, including compliance policy and procedural enhancements, improved internal controls, audits, improved compliance training and potentially employee discipline, any of which could have an adverse impact on our business, results of operations and financial condition including our
liquidity. Any investigation of any potential violations of the FCPA or other anti-corruption laws by U.S. or foreign authorities also could have an adverse impact on our business, results of operations and financial condition.
Certain foreign companies, including some of our competitors, are not subject to prohibitions as strict as those under the FCPA or, even if subjected to strict prohibitions, such prohibitions may be laxly enforced in practice. If our competitors engage in corruption, extortion, bribery, pay-offs, theft or other fraudulent practices, they may receive preferential treatment from personnel of some companies, giving our competitors an advantage in securing business, or from government officials, who might give them priority in obtaining new licenses, which would put us at a disadvantage.
Legal compliance with import and export controls, as well as with sanctions, in the United States and other countries, is complex, and compliance restrictions and expenses could materially and adversely impact our revenue and supply chain.
We are subject to a variety of import laws, export controls and economic sanctions laws and regulations, including rule changes, and evolving enforcement practices. Changes in import and export control or trade sanctions laws may restrict our business practices and affect our ability to supply our products to various countries and/or to various customers, including cessation of business activities in sanctioned countries or with sanctioned entities, and may result in claims for breach of existing contracts and modifications to existing compliance programs and training schedules. Violations of the applicable export or import control, or economic sanctions laws and regulations, such as an export to an embargoed country, or to a denied party, or the export of a product without the appropriate governmental license, may result in penalties, including fines, debarments from export privileges, and loss of authorizations needed to conduct aspects of our international business, and may harm our ability to enter into contracts with our customers who have contracts with the U.S. government. A violation of the laws and regulations enumerated above could have an adverse effect on our business, results of operations and financial condition. Additionally, we require our sales channel partners in certain non-U.S. jurisdictions to comply with certain standards as part of our trade compliance program and regularly review our partners’ performance of their compliance obligations. As part of these reviews, it is possible we may discover that certain partners do not meet our standards, and we may be required to terminate agreements with any non-compliant partners. Any such actions could materially and adversely impact our ability to do business in jurisdictions where we are unable to enter into agreements with alternative partners that meet our compliance standards. This in turn could materially and adversely impact our competitive position in such jurisdictions, as well as on our business, results of operations and financial condition, including our cash flows.
Without limiting the generality of the preceding paragraph, political and diplomatic developments between the United States and the People’s Republic of China could have a significant impact on our sales and business operations in China and with our Chinese customers, including projects outside of China for which Chinese customers are prime contractors or sub-contractors. In the past four years, the United States Government has imposed new export control restrictions and special export licensing requirements on many major Chinese companies, including certain Chinese companies that have previously been our customers. Further actions by the United States Government to restrict exports to China and Chinese entities could significantly affect our continuing ability to do business in China.
Our business may also be affected by new sanctions and export controls targeting Russia and other responses to Russia's invasion of Ukraine.
As a result of Russia's invasion of Ukraine, the United States, the United Kingdom and the European Union governments, among others, have developed coordinated sanctions and export-control measure packages.
Based on the public statements to date, these packages include:
•comprehensive financial sanctions against major Russian banks (including SWIFT cut off);
•additional designations of Russian individuals with significant business interests and government connections;
•designations of individuals and entities involved in Russian military activities; and
•enhanced export controls and trade sanctions targeting Russia's imports of technological goods as a whole, including potentially tighter controls on exports and reexports of dual-use items, stricter licensing policy with respect to issuing export licenses, and/or increased use of "end-use" controls to block or impose licensing requirements on exports.
We currently have existing contracts and an order backlog with Russian customers and customers in other countries whose contracts with us may be financed by, or involve, Russian entities. The imposition of enhanced export controls and economic sanctions on transactions with Russia and Russian entities by the United States, the United Kingdom, and/or the European Union could prevent us from performing existing contracts, recognize revenue from our backlog, pursuing new
business opportunities and/or receiving payment for products already supplied and services already performed with Russian and other customers. In addition, even if a Russian entity is not formally subject to sanctions, customers of such Russian entity may decide to reevaluate, or cancel projects with such entity, and such actions could have a similar impact on us as if sanctions were applied directly as described above. Depending on the extent and breadth of new sanctions or export controls that may be imposed against Russia, it is possible that our business, results of operations and financial condition could be materially and adversely affected.
Any actual or perceived failure to comply with evolving data privacy and data security laws and regulations in the jurisdictions where we operate, both inside and outside of the United States, could lead to government enforcement actions (which could include civil or criminal penalties), private litigation or adverse publicity and could materially and adversely affect our business.
Privacy and data security have become significant issues in the United States, Europe and in many other jurisdictions where we conduct our operations. Our collection, processing, distribution, and storage of personal information is subject to a variety of laws and regulations both in the United States and abroad, which could limit the way we market and provide our products and services. Compliance with these privacy and data security requirements is rigorous and time-intensive and may increase our cost of doing business and, despite these efforts, there is a risk that we fail to comply and may become subject to government enforcement actions, fines and penalties, litigation and reputational harm, which could materially and adversely affect our business, results of operations and financial condition. In addition, the regulatory framework for the handling of personal and confidential information is rapidly evolving and is likely to remain uncertain for the foreseeable future as new privacy laws are being enacted globally and existing laws are being updated and strengthened.
For example, in May 2018, the General Data Protection Regulation (“GDPR”) superseded prior European Union data protection legislation, and it imposes more stringent European Union data protection requirements, and provides for greater penalties for noncompliance. Under the GDPR, fines of up to 20 million euro or up to 4% of the annual global turnover of the infringer, whichever is greater, could be imposed. The GDPR is wide-ranging in scope and imposes numerous additional requirements on companies that process personal data, including imposing special requirements in respect of the processing of personal data, requiring that consent of individuals to whom the personal data relates is obtained in certain circumstances, requiring additional disclosures to individuals regarding data processing activities, requiring that safeguards are implemented to protect the security and confidentiality of personal data, creating mandatory data breach notification requirements in certain circumstances, and requiring that certain measures (including contractual requirements) are put in place when engaging third-party processors. The GDPR also provides individuals with various rights in respect of their personal data, including rights of access, erasure, portability, rectification, restriction and objection.
Further, the United Kingdom’s vote in favor of exiting the European Union, often referred to as Brexit, and ongoing developments in the United Kingdom have created uncertainty with regard to data protection regulation in the United Kingdom. As of January 1, 2021, and the expiry of transitional arrangements agreed to between the United Kingdom and the European Union, data processing in the United Kingdom is governed by a United Kingdom version of the GDPR (combining the GDPR and the Data Protection Act 2018), exposing us to two parallel regimes, each of which potentially authorizes similar fines and other potentially divergent enforcement actions for certain violations. With respect to transfers of personal data from the European Economic Area (“EEA”) to the United Kingdom, the European Commission adopted an adequacy decision for the United Kingdom on June 28, 2021, finding the United Kingdom ensures an adequate level of data protection. Following the adoption of the adequacy decision, there will be increasing scope for divergence in application, interpretation and enforcement of the data protection law as between the United Kingdom and EEA. Other countries have also passed or are considering passing laws requiring local data residency or restricting the international transfer of data.
Other jurisdictions outside the European Union are similarly introducing or enhancing privacy and data security laws, rules and regulations, which could increase our compliance costs and the risks associated with noncompliance. For example, California recently enacted the California Consumer Privacy Act (“CCPA”) which creates new individual privacy rights for California consumers (as defined in the law) and places increased privacy and security obligations on companies handling personal information of consumers or households. The CCPA, which went into effect on January 1, 2020, requires covered companies to provide new disclosure to consumers about such companies’ data collection, use and sharing practices, provide methods for such consumers to access and delete their personal information, with exceptions, as well as allowing consumers to opt-out of certain sales or transfers of their personal information. The CCPA provides for civil penalties for violations and further provides consumers with a new private right of action in the event of a data breach involving certain sensitive information as a result of the business’ failure to implement reasonable security measures. This private right of action may increase the likelihood of, and risks associated with, data breach litigation. The California Attorney General’s enforcement authority under the CCPA became effective July 1, 2020, and it remains unclear how various provisions of the CCPA will be interpreted and enforced. As currently written, the CCPA impacts certain of our business activities and exemplifies the vulnerability of our business to the evolving regulatory environment related to personal information. A
ballot initiative from privacy rights advocates intended to augment and expand the CCPA called the California Privacy Rights Act (“CPRA”) was passed in November 2020 and will take effect in January 2023 (with a look back to January 2022). The CPRA significantly modifies the CCPA, including by imposing additional obligations on covered companies and expanding consumers’ rights with respect to certain sensitive personal information, potentially resulting in further uncertainty and requiring us to incur additional costs and expenses in an effort to comply. The CPRA also creates a new state agency that will be vested with authority to implement and enforce the CCPA and the CPRA. In addition, all 50 states have laws including obligations to provide notification of security breaches of computer databases that contain personal information to affected individuals, state officers and others. Aspects of the CCPA, the CPRA, and other laws and regulations relating to data protection, privacy, and information security, as well as their enforcement, remain unclear, and we may be required to modify our practices in an effort to comply with them.
We cannot yet fully determine the impact these or future laws, rules, and regulations concerning data privacy and security may have on our business or operations. These laws, rules and regulations may be inconsistent from one jurisdiction to another, subject to differing interpretations and may be interpreted to conflict with our practices. Additionally, we may be bound by contractual requirements applicable to our collection, use, processing and disclosure of various types of data, including personal information, and may be bound by, or voluntarily comply with, self-regulatory or other industry standards relating to these matters. Compliance with U.S. and international privacy and data security laws and regulations could require us to take on more onerous obligations in our contracts and restrict our ability to collect, use and disclose data. Because the interpretation and application of data protection laws, regulations, standards and other obligations are still uncertain, and often contradictory and in flux, it is possible that the scope and requirements of these laws may be interpreted and applied in a manner that is inconsistent with our practices and our efforts to comply with the evolving data protection rules may be unsuccessful. Failure to comply with U.S. and international privacy and data security laws and regulations could result in government enforcement actions (which could include civil or criminal penalties), private litigation or adverse publicity and could negatively affect our results of operations and business. Claims that we have violated individuals’ privacy rights, failed to comply with privacy and data security laws, or breached our contractual obligations, even if we are not found liable, could be expensive and time consuming to defend and could result in adverse publicity that could increase our operation costs, impact our financial performance and adversely affect enrollments.
Our ability to compete successfully and achieve future growth will depend on our ability to obtain, maintain, protect, defend and enforce our intellectual property and to operate without infringing, misappropriating or otherwise violating the intellectual property of others.
Our intellectual property, including our design, engineering, manufacturing and testing know-how, is an essential asset of our business. Failure to adequately protect our intellectual property rights could result in our competitors or other third parties offering similar products and services, potentially resulting in the loss of our competitive advantage and a decrease in our revenue, which would adversely affect our business, results of operations and financial condition. We attempt to protect our intellectual property rights through patents, trademarks, copyrights, trade secret laws, non-disclosure agreements, confidentiality procedures, employee disclosure and invention assignment agreements and other contractual provisions. We cannot guarantee that any of our pending patent applications or other applications for intellectual property registrations will be issued or granted or that our existing and future intellectual property rights will be sufficiently broad to protect our proprietary technology.
While a presumption of validity exists with respect to United States patents issued to us, there can be no assurance that any of our patents, patent applications, or other intellectual property rights will not be, in whole or in part, opposed, contested, challenged, invalidated, circumvented, designed around, or rendered unenforceable. If we fail to obtain issuance of patents or registration of other intellectual property, or our patent claims or other intellectual property rights are rendered invalid or unenforceable, or narrowed in scope, pursuant to, for example, judicial or administrative proceedings including re-examination, post-grant review, inter partes, interference, opposition, or derivation proceedings, the coverage of patents and other intellectual property rights afforded our products could be impaired. Even if we are to obtain issuance of further patents or registration of other intellectual property, such intellectual property could be subjected to attacks on ownership, validity, enforceability, or other legal attacks. Any such impairment or other failure to obtain sufficient intellectual property protection could materially and adversely affect our business, results of operations and financial condition, including forcing us to, among other things, rebrand or re-design our affected products. Moreover, our patents and patent applications may only cover particular aspects of our products, and competitors and other third parties may be able to circumvent or design around our patents. Competitors may develop and obtain patent protection for more effective technologies, designs or methods. There can be no assurance that third parties will not create new products or methods that achieve similar or better results without infringing upon patents we own. If these developments were to occur, it could have an adverse effect on our business, results of operations and financial condition.
While we generally seek or apply for patent protection as and if we deem appropriate, based on the then-current facts and circumstances, we also rely upon unpatented proprietary radiation detection expertise, continuing technological innovation
and other trade secrets some of which is licensed from third parties, to develop and maintain our competitive position. We seek to enter into confidentiality agreements with our employees and third parties who have access to our confidential or proprietary information; however, we may fail to enter into such agreements with all parties who have access to our confidential information, such agreements are often limited in duration and such agreements could be breached, and therefore they may not provide meaningful protection for our trade secrets, including our proprietary radiation detection and measurement expertise. Similarly, while we seek to enter into agreements with all of our employees and contractors who develop intellectual property during their engagement with us to assign the rights in such intellectual property to us, we may fail to enter into such agreements with all relevant employees and contractors, such agreements may be breached or may not be self-executing, and we may be subject to claims that such employees or contractors misappropriated relevant rights from their previous employers.
We cannot guarantee that the steps we have taken to protect our intellectual property will be adequate to prevent infringement of our intellectual property rights or misappropriation of our technology, trade secrets or know-how. It is possible that our efforts to protect our intellectual property rights may not:
•prevent others from obtaining knowledge of our trade secrets through independent development or other access by legal means;
•prevent our competitors or other third parties from independently developing similar products, duplicating our products or designing around the patents owned by us;
•prevent third-party patents from having an adverse effect on our ability to do business;
•provide adequate protection for our intellectual property rights;
•prevent disputes with third parties regarding ownership of, or exclusive rights to, our intellectual property;
•prevent disclosure of our trade secrets and know-how to third parties or into the public domain;
•prevent the challenge, invalidation or circumvention of our existing patents;
•result in patents that lead to commercially viable products or provide competitive advantages for our products; and
•result in issued patents and registered trademarks from any of our pending applications.
The laws of foreign countries also may not adequately protect our intellectual property rights. Many U.S. companies have encountered substantial infringement, misappropriation or other violations of their intellectual property rights in foreign countries. Furthermore, because filing, prosecuting, maintaining, and defending our intellectual property in all countries throughout the world would be prohibitively expensive we have not applied for patent protection or trademark or other intellectual property registrations in all jurisdictions in which we currently, or may in the future, operate. Because we conduct a substantial portion of our operations and a majority of our sales have been outside of the United States, we have significant exposure to foreign intellectual property risks.
Others have in the past attempted, and may in the future attempt, to copy or otherwise obtain and use our intellectual property without our consent. For example, our customers or their end users’ customers may attempt to copy or otherwise obtain and use our intellectual property without our consent. Monitoring the unauthorized use of our intellectual property is difficult and we may fail to identify instances where a third party is infringing, misappropriating or otherwise violating our intellectual property. If we fail to protect our intellectual property rights adequately, we may lose an important advantage in the markets in which we compete.
We are currently party to, and may in the future initiate, litigation against one or more third parties to preserve or enforce our intellectual property rights or to challenge the validity and scope of proprietary rights asserted by others, and we could face counterclaims. Such efforts may be insufficient or ineffective, and any of our intellectual property rights may be challenged, which could result in them being narrowed in scope or declared invalid or unenforceable. Furthermore, any such legal disputes we may initiate with our customers or companies with whom we have manufacturing relationships could substantially harm our relationships and sales. An adverse outcome in any such proceeding could subject us to significant liability for damages or invalidate our proprietary rights. Such litigation could result in significant expense to us and divert the efforts of our technical and management personnel, whether or not such litigation is ultimately determined in our favor. Further, adequate remedies may not be available in the event of an unauthorized use or disclosure of our trade secrets and manufacturing expertise. Any of the foregoing could materially and adversely affect our business, results of operations and financial condition.
We may need to defend ourselves against third-party claims that we are infringing, misappropriating or otherwise violating others’ intellectual property rights, which could divert management’s attention, cause us to incur significant costs and prevent us from selling or using the technology to which such rights relate.
Our commercial success depends in part on avoiding infringement, misappropriation or other violations of the intellectual property and proprietary rights of third parties and other intellectual property-related disputes. There may be intellectual property rights held by others, including issued or pending patents and registered trademarks, that cover significant aspects of our technologies, products or services, and we cannot be sure that we are not infringing or violating, and have not infringed or violated, any third-party intellectual property rights. From time to time, third parties have claimed and may claim in the future that we have infringed upon, misappropriated or misused their proprietary rights, and we may be unaware of existing third-party intellectual property rights that we may be infringing.
Any of these events or claims could result in litigation. Such litigation could result in significant expense to us and divert the efforts of our technical and management personnel, whether or not such litigation is ultimately determined in our favor. In the event of an adverse result in such litigation, we could be required to pay substantial damages, cease the manufacture, use and sale of certain products, expend significant resources to develop or acquire non-infringing technology, discontinue the use of certain processes, obtain licenses to use the infringed technology or indemnify our customers. Product development or obtaining a license would likely result in significant expense to us and divert the efforts of our technical and management personnel. We cannot assure you that we would be successful in such development or acquisition or that such licenses would be available on reasonable terms, or at all. If we cannot license or develop a non-violating alternative, we would be forced to limit or stop sales of our offerings and may be unable to effectively compete. Moreover, there could be public announcements of the results of hearings, motions or other interim proceedings or developments and if securities analysts or investors perceive these results to be negative, it could have a substantial adverse effect on the price of our stock. Any of these results would materially and adversely affect our business, results of operations and financial condition.
Our use of “open source” software could negatively affect our ability to sell our products and subject us to possible litigation.
A portion of our products incorporate so-called “open source” software, and we may incorporate additional open source software in the future. Open source software is generally licensed by its authors or other third parties under open source licenses. If we fail to comply with these licenses, we may be subject to certain conditions, including requirements that we offer our products that incorporate the open source software for no cost, that we make available source code for modifications or derivative works we create based upon, incorporating or using the open source software and/or that we license such modifications or derivative works under the terms of the particular open source license. If an author or other third party that distributes such open source software were to allege that we had not complied with the conditions of one or more of these licenses, we could be required to incur significant legal expenses defending against such allegations and could be subject to significant damages, enjoined from the sale of our products that contained the open source software and required to comply with the foregoing conditions, which could disrupt the distribution and sale of some of our products and adversely affect our business, results of operations and financial condition.
Our obligations to indemnify our customers for the infringement, misappropriation or other violation by our products of the intellectual property rights of others could require us to pay substantial damages and impose other costs and fees.
We currently have in effect, and may in the future enter into, agreements in which we agree to defend, indemnify and hold harmless our customers or suppliers from damages and costs that may arise from the infringement, misappropriation or other violation by our products of third-party patents, trademarks or other proprietary rights. We may periodically have to respond to claims and initiate or participate in litigation in connection with these indemnification obligations, which may result in our paying substantial damages. Such litigation could result in significant expense to us and divert the efforts of our technical and management personnel, whether or not such litigation is ultimately determined in our favor. Our insurance does not cover intellectual property infringement. Any of the foregoing could materially and adversely affect our business, results of operations and financial condition.
We could incur substantial costs as a result of violations of, or liabilities under, environmental laws.
Our operations and properties are subject to a variety of federal, state, local and foreign environmental, health and safety laws and regulations governing, among other things, air emissions, wastewater discharges, management and disposal of hazardous, non-hazardous and radioactive materials and waste and remediation of releases of hazardous materials. Compliance with environmental requirements could require us to incur significant operating or capital expenditures or result in significant restrictions on our operations. Our failure to comply with these environmental, health and safety laws and regulations, including failing to obtain any necessary permits, could cause us to incur substantial civil or criminal fines
or penalties or enforcement actions, including regulatory or judicial orders enjoining or curtailing our operations or requiring us to conduct or fund remedial or corrective measures, install pollution control equipment or perform other actions. Under certain of these laws and regulations, we may be subject to joint and several liability for environmental investigations and cleanups, including at properties that we currently or previously owned or operated, or at sites at which waste we generated was disposed, even if the contamination was not caused by us or was legal at the time it occurred. The future identification of presently unidentified environmental conditions, more vigorous enforcement by regulatory agencies, enactment of more stringent laws, regulations or permit requirements, including relating to climate change, or other unanticipated events may arise in the future and give rise to material environmental liabilities and related costs or adversely impact the market for our products, which could materially and adversely affect our business, results of operations and financial condition.
A European Union (“EU”) directive relating to the restriction of hazardous substances in electrical and electronic equipment (“RoHS Directive”) and an EU directive relating to waste electrical and electronic equipment (“WEEE Directive”) have been and are being implemented in EU member states. Among other things, the RoHS directive restricts the use of certain hazardous substances in the manufacture of electrical and electronic equipment and the WEEE directive requires producers of electrical goods to be responsible for the collection, recycling, treatment and disposal of these goods. In addition, laws similar to the RoHS and WEEE directives were passed in China in 2006 and South Korea in 2007. Governments in other countries and states, including the United States, have implemented or are considering implementing similar laws or regulations.
In addition, a regulation regarding the registration, authorization and restriction of chemical substances in industrial products (“REACH”) became effective in the EU in 2007. REACH and other regulations require us or our suppliers to substitute certain chemicals contained in our products with substances the EU considers less dangerous. We cannot assure you that REACH or similar regulations will not materially affect us in the future.
The costs associated with complying with future laws and regulations could include costs associated with modifying, requalifying or reformulating our products, recycling and other waste processing costs, or legal and regulatory costs and insurance costs. We have recorded in the past and may be required to record in the future additional expenses for costs associated with compliance with regulations. The costs of complying with future environmental and worker health and safety laws and regulations could materially and adversely affect our business, results of operations and financial condition.
We do not control our suppliers, customers or business partners, and facts or circumstances that may occur as a result of their actions or omissions could harm our reputation and sales.
We do not control our suppliers, customers or partners, or their environmental or other practices. A violation of environmental or other laws by our suppliers, other customers or partners, or an environmental or public health incident at customer locations, including, for example, a nuclear incident at a facility to which we supplied equipment or that we serviced, or any failure of these third parties to follow generally accepted ethical business practices, could create negative publicity and harm our reputation. In addition, we may be required to seek alternative suppliers or partners if these violations or failures were to occur. We do not inspect or audit compliance of our suppliers, customers or partners with these laws or practices, and we do not require our suppliers, customers or partners to comply with a formal code of conduct. Any conduct or actions that our suppliers could take could reduce demand for our products, harm our ability to meet demand or harm our reputation, brand image, business, results of operations and financial condition.
Some of our workforce is represented by labor unions in the United States and by works councils and trade unions in the EU, and are covered by collective bargaining agreements in connection with such representations. Labor group representation may lead to work stoppages that could materially and adversely affect our business, including as a result of a failure to renegotiate a collective bargaining agreement.
As of December 31, 2021, approximately 36 of our U.S. employees were unionized, or 1.4% of our employees globally, and the majority of our EU employees are members of, or are represented by, works councils or trade unions and are covered by collective bargaining agreements. In addition, employees who are not currently members of, or otherwise represented by, labor organizations may seek such membership or representation, as applicable, in the future. Since 1988, we have experienced only two work stoppages, each time at our facility in Lamanon, France that lasted less than half a day. We may experience work stoppages or other labor disturbances in the future, including in connection with the renegotiation of collective bargaining agreements as they expire, which could adversely affect our business. We cannot predict how stable our relationships will be or whether we will be able to satisfy union or works council requirements without impacting our business, results of operations and financial condition. Union and works council rules may limit our flexibility to respond to changing market conditions and the application of these rules could harm our business. The unions and works councils may also limit our flexibility in dealing with our workforce. Work stoppages and instability in our relationships could negatively impact the timely production of our products, which could strain relationships with
customers and cause a loss of revenue that would adversely affect our results of operations. Additionally, any renegotiation of current collective bargaining agreements may result in terms that are less favorable to us.
The elimination or any modification of the Price-Anderson Act’s indemnification authority could have adverse consequences for our business.
In the United States, the Atomic Energy Act of 1954, as amended (“AEA”), comprehensively regulates the manufacture, use and storage of radioactive materials. Section 170 of the AEA, which is known as the Price-Anderson Act, supports the nuclear services industry by offering broad indemnification for third-party public liability claims arising from a nuclear accident occurring at any commercial NPP in the United States. The Act channels the nuclear liability to the licensee plant operator and provides omnibus coverage for all firms that contribute in any way to the design, construction or operation of a licensed reactor, including vendors, contractors, suppliers, engineers, consulting firms, and transporters. The indemnification authority of the Nuclear Regulatory Commission (“NRC”), and Department of Energy (“DOE”) under the Price-Anderson Act has been extended by Congress numerous times since enactment in 1957, including most recently through 2025 by the Energy Policy Act of 2005. Extension is often largely uncontroversial, although it has met opposition at times due primarily to the view that the Act is a subsidy for the nuclear energy industry. Some of our customers are covered by the DOE indemnification provisions of the Price-Anderson Act for contractors. In addition, other jurisdictions have similar nuclear liability laws with indemnification authority to protect suppliers. If the nuclear liability and indemnification authority in the United States or other countries is eliminated or adversely modified in the future, our business could be adversely affected if the owners and operators of NPPs cancel or delay plans to build new plants or curtail the operations of existing plants. Although it is unlikely that the nuclear liability financial protection authority under the Price-Anderson Act would be completely abolished, some aspects of the Act could be changed during future reauthorizations.
Certain of our products and software are subject to ongoing regulatory oversight by the FDA or equivalent regulatory agencies in international markets and if we are not able to obtain or maintain the necessary regulatory approvals we may not be able to continue to market and sell such products which may materially and adversely affect our business.
The FDA regulates virtually all aspects of a medical device design, development, testing, manufacturing, labeling, storage, record keeping, adverse event reporting, sale, promotion, distribution and shipping. Before a new medical device, including a new intended use, indication, or claim for an existing product, can be marketed in the United States, it must first receive either premarket approval or 510(k) clearance from the FDA, unless an exemption exists. Either process can be expensive, lengthy and unpredictable. The FDA’s 510(k) clearance process generally takes from three to twelve months, but it can last longer. The process of obtaining premarket approval is much more costly and uncertain than the 510(k) clearance process and it generally takes from one to three years, or even longer, from the time the application is filed with the FDA. Additionally, outside of the United States, our products are subject to clearances and approvals by foreign FDA counterparts. In order to market our products internationally, we must obtain licenses or approvals from these governmental agencies, which could include local requirements, safety standards, testing or certifications, and can be time consuming, burdensome and uncertain. Despite the time, effort and cost, there can be no assurance that a particular device or a modification of a device will be approved or cleared by the FDA, or any foreign governmental agency in a timely fashion, if at all. Even if we are granted regulatory clearances or approvals, they may include significant limitations on the indicated uses of the product, which may limit the market for those products, and how those products can be promoted.
Medical devices may only be marketed for the indications for which they are approved or cleared. The FDA and other foreign governments also may change their policies, adopt additional regulations, or revise existing regulations, each of which could prevent or delay approval or clearance of our device, or could impact our ability to market our currently approved or cleared devices. We are also subject to medical device reporting regulations, which require us to report to the FDA and other international governmental agencies if our products cause or contribute to a death or a serious injury, or malfunction in a way that would likely cause or contribute to a death or a serious injury. Further, we are subject to the QSR in the United States and ISO 13485 certification in many international markets, compliance with which is necessary to receive FDA and other international clearances or approvals to market new products, and is necessary for us to be able to continue to market a cleared or approved product in the United States or globally. After a product is placed in the market, we are also subject to oversight by the FDA and Federal Trade Commission related to the advertising and promotion of our products to ensure our claims are consistent with our regulatory clearances, that there is scientific data to substantiate our claims, and that our advertising is not false or misleading. Our products are also subject to state regulations and various international laws and regulations.
A component of our strategy is to continue to upgrade products such as SunCHECK, SunScan 3D or Lynx. Our previous upgrades required 510(k) clearance and international registration before we were able to offer them for sale. We expect our future upgrades will similarly require 510(k) clearance or approval; however, future upgrades may be subject to substantially more time consuming data generation requirements and uncertain premarket approval or clearance processes.
If we were required to use the premarket approval process for future products or product modifications, it could delay or prevent release of the proposed products or modifications, which could harm our business.
The FDA requires device manufacturers to make their own determination of whether or not a modification requires an approval or clearance; however, the FDA can review a manufacturer’s decision not to submit for additional approvals or clearances. Any modification to an FDA approved or cleared device that would significantly affect its safety or efficacy or that would constitute a major change in its intended use would require a new premarket approval or 510(k) clearance. We cannot ensure that the FDA will agree with our decisions not to seek approvals or clearances for particular device modifications or that we will be successful in obtaining premarket approvals or 510(k) clearances for modifications in a timely fashion, if at all.
We have obtained 510(k) clearance for SunCHECK to be used as an integrated patient quality assurance, machine quality assurance and data management workflow management application for radiation therapy professionals. We have made modifications to SunCHECK in the past and may make additional modifications in the future that we believe do not or will not require additional approvals or clearances. If the FDA disagrees, based on new finalized guidance and requires us to obtain additional premarket approvals or 510(k) clearances for any modifications to SunCHECK and we fail to obtain such approvals or clearances or fail to secure approvals or clearances in a timely manner, we may be required to cease manufacturing and marketing the modified device or to recall such modified device until we obtain FDA approval or clearance and we may be subject to significant regulatory fines or penalties.
The FDA and similar governmental authorities in other countries in which we market and sell our products have the authority to require the recall of our products in the event of material deficiencies or defects in design, manufacture or labeling, and from time to time we have conducted and may in the future conduct such recalls.
For example, in August 2021, Mirion Technologies (Biodex), Inc. initiated a voluntary recall of certain versions of AtomLab 500 and AtomLab500 Plus, which was reported to the FDA. The AtomLab 500 is a radioisotope dose calibrator used to measure radiopharmaceuticals prior to administration to a patient and versions 2.0.00 through 2.0.08 contained a software error affecting only the custom isotope list (the 99 commonly used default isotopes were not affected). The recall affected 1,256 units. The software error was corrected in Version 2.0.10 of the software released in June 2021.
A government mandated recall, or a voluntary recall by us, could occur as a result of component failures, manufacturing errors or design defects, including defects in labeling and user manuals. Any recall could divert management’s attention, cause us to incur significant expenses, generate negative publicity, harm our reputation with customers, negatively affect our future sales and business, require redesign of our products, and harm our operating results. In these circumstances, we may also be subject to significant enforcement action. If any of these events were to occur, our ability to introduce new or enhanced products in a timely manner would be adversely affected, which in turn would harm our future growth.
We are subject to federal, state, local and international laws and regulations related to healthcare, the violation of which could result in substantial penalties and harm our business in the medical end market.
Our operations are subject to several laws and regulations governing interactions with healthcare providers. The Medicare and Medicaid “anti-kickback” laws, and similar state laws, prohibit soliciting, offering, paying or accepting any payments or other remuneration that is intended to induce any individual or entity to either refer patients to or purchase, lease or order, or arrange for or recommend the purchase, lease or order of, healthcare products or services for which payment may be made under federal and state healthcare programs, such as Medicare and Medicaid. Such laws impact our sales, marketing and other promotional activities by reducing the types of financial arrangements we may have with our customers, potential customers, marketing consultants and other service providers. They particularly impact how we structure our sales offerings, including discount practices, customer support, product loans, education and training programs, physician consulting, research grants and other service arrangements. Many of these laws are broadly drafted and are open to a variety of interpretations, making it difficult to determine with any certainty whether certain arrangements violate such laws, even if statutory safe harbors are available.
In addition to such anti-kickback laws, federal and state “false claims” laws generally prohibit the knowing filing or causing the filing of a false claim, or the knowing use of false statements to obtain payment from government payors. Although we do not submit claims directly to payors, manufacturers can be held liable under these laws if they are deemed to “cause” the submission of false or fraudulent claims by providing inaccurate billing or coding information to customers, or through certain other activities, including promoting products for uses or indications that are not approved by the FDA.
We are also subject to federal and state physician self-referral laws. The federal Ethics in Patient Referrals Act of 1989, commonly known as the Stark Law, prohibits, subject to certain exceptions, physician referrals of Medicare and Medicaid patients to an entity providing certain “designated health services” if the physician or an immediate family member has any
financial relationship with the entity. The Stark Law also prohibits the entity receiving the referral from billing any good or service furnished pursuant to an unlawful referral. Various states have corollary laws to the Stark Law, including laws that require physicians to disclose any financial interest they may have with a healthcare provider to their patients when referring patients to that provider. Both the scope and exceptions for such laws vary from state to state.
If our past or present operations are found to be in violation of any of these “anti-kickback,” “false claims,” “self-referral” or other similar laws in foreign jurisdictions, we may be subject to the applicable penalty associated with the violation, which may include significant civil and criminal penalties, damages, fines, imprisonment and exclusion from healthcare programs. The impact of any such violations may lead to curtailment or restructuring of our operations, which could adversely affect our ability to operate our business and our financial results.
There are a number of federal and state laws protecting the confidentiality of certain patient health information, including patient records, and restricting the use and disclosure of that protected information. In particular, the U.S. Department of Health and Human Services (“HHS”), has promulgated patient privacy rules under the Health Insurance Portability and Accountability Act (“HIPAA”). These privacy rules protect medical records and other personal health information of patients by limiting their use and disclosure, giving patients the right to access, amend and seek accounting of their own health information and limiting most uses and disclosures of health information to the minimum amount reasonably necessary to accomplish the intended purpose. The HIPAA privacy standard was amended by the Health Information Technology for Economic and Clinical Health Act, enacted as part of the American Recovery and Reinvestment Act of 2009. Although we are not a “covered entity” under HIPAA, we are considered a “business associate” of certain covered entities and, as such, we are directly subject to HIPAA, including its enforcement scheme and inspection requirements, and are required to implement policies, procedures as well as reasonable and appropriate physical, technical and administrative security measures to protect individually identifiable health information we receive from covered entities. Our failure to protect health information received from customers in compliance with HIPAA or other laws could subject us to civil and criminal liability to the government and civil liability to the covered entity, could result in adverse publicity, and could harm our business and impair our ability to attract new customers.
The Sunshine Act, which was enacted by Congress as part of the Patient Protection and Affordable Care Act on December 14, 2011, requires each applicable manufacturer, which includes medical device companies, to track and report to the federal government on an annual basis all payments and other transfers of value from such applicable manufacturer to U.S. licensed physicians and teaching hospitals as well as physician ownership of such applicable manufacturer’s equity, in each case subject to certain statutory exceptions. Furthermore, on October 25, 2018, President Trump signed into law the “Substance Use-Disorder Prevention that Promoted Opioid Recovery and Treatment for Patients and Communities Act” which in part (under a provision entitled “Fighting the Opioid Epidemic with Sunshine”) extends the reporting and transparency requirements for physicians in the Physician Payments Sunshine Act to physician assistants, nurse practitioners, clinical nurse specialists, certified registered nurse anesthetists, and certified nurse midwives (with reporting requirements going into effect in 2022 for payments made in 2021). Such data will be made available by the government on a publicly searchable website. Failure to comply with the data collection and reporting obligations imposed by the Sunshine Act can result in civil monetary penalties ranging from $1,000 to $10,000 for each payment or other transfer of value that is not reported (up to a maximum of $150,000 per reporting period) and from $10,000 to $100,000 for each knowing failure to report (up to a maximum of $1 million per reporting period). In addition, we are subject to similar state and foreign laws related to the tracking and reporting of payments and other transfers of value to healthcare professionals, the violation of which could, among other things, result in civil monetary penalties and adversely impact our reputation and business.
Healthcare reform legislation could materially and adversely affect demand for our products, our revenue and our financial condition.
In March 2010, the Patient Protection and Affordable Care Act, as amended by Health Care and Education Reconciliation Act, collectively referred to as the ACA were signed into law. The ACA includes a large number of health related provisions, including expanding Medicaid eligibility, requiring most individuals to have health insurance, establishing new regulations on health plans, establishing health insurance exchanges, requiring manufacturers to report payments or other transfers of value made to physicians and teaching hospitals, modifying certain payment systems to encourage more cost-effective care and a reduction of inefficiencies and waste and including new tools to address fraud and abuse. The laws also include a decrease in the annual rate of inflation for Medicare payments to hospitals and the establishment of an independent payment advisory board to suggest methods of reducing the rate of growth in Medicare spending. The expansion in the government’s role in the U.S. healthcare industry may result in decreased profits to us, lower reimbursement by third-party payors for our products, or reduced volume of medical procedures conducted with our products, all of which could materially and adversely affect our business, results of operations and financial condition. The federal government may take further action regarding the ACA, including, but not limited to, repeal or replacement action. Most recently, the Tax Cuts and Jobs Act was signed into law in December 2017, which, among other things, removed
penalties for not complying with the individual mandate to carry health insurance. Additionally, all or a portion of the ACA and related subsequent legislation may be modified, repealed or otherwise invalidated through judicial challenge, which could result in lower numbers of insured individuals, reduced coverage for insured individuals and adversely affect our business. We continue to monitor the impact that the ACA may have on our business.
In addition, since the adoption of the Affordable Care Act, other legislation designed to keep federal healthcare costs down has been proposed or passed. For example, under the sequestration required by the Budget Control Act of 2011, as amended by the American Taxpayer Relief Act of 2012, Medicare payments for all items and services under Parts A and B incurred on or after April 1, 2013 have been reduced by up to 2%. Future federal legislation may impose further limitations on the coverage or amounts of reimbursement available for our products from governmental agencies or third-party payors. These limitations could have a negative impact on the demand for our products and services, and therefore on our financial position and results of operations.
Since the enactment of the ACA, the Centers for Medicare and Medicaid Services (“CMS”) continues its efforts to move away from fee-for-service payments for furnishing items and services in Medicare. In the past several rulemaking cycles, CMS has increased packaging policies and created larger payment bundles across the Medicare Hospital Outpatient Prospective Payment System (“OPPS”). One example is CMS’s expansion of Comprehensive Ambulatory Payment Classifications, under which payment for adjunctive and secondary items, services and procedures are packaged into the most costly primary procedure at the claim level. Beyond the OPPS, CMS’s Innovation Center has launched a number of alternative payment model (“APM”) demonstrations that involve episode-based (i.e. bundled) payment. Since 2011, for example, Center for Medicare and Medicaid Innovation (“CMMI”) has created and is in the process of creating major federal initiatives to test episode-based payments, such as the Bundled Payments for Care Improvement, Oncology Care Model, Specialty Practitioners Payment Model Opportunities. More recently, CMMI proposed a Radiation Oncology Model, which would mandate selected radiotherapy providers to participate in a prospective, episode-based payments model where payment is based on a patient’s diagnosis as opposed to the traditional volume-based fee-for service payment model. It is unclear what impact, if any, such initiatives will have on our business and operating results, but uncertainties surrounding the implementation of these payment models could pause or otherwise delay the purchase of our products by our customers and any resulting decrease in reimbursement to our customers may result in reduced demand for our services.
Furthermore, the Patient Access and Medicare Protection Act of 2015 froze payment for some radiation therapy delivery and related services, and requires CMS to provide a report to the U.S. Congress on the development of an APM for radiation therapy services provided in non-facility settings. While these types of payment packaging policies and episode-based payments may impact reimbursement for overall patient care, including items and services furnished to patients, they also create incentives for providers to carefully assess the value proposition of technology purchases and uses. The impacts of these payment and delivery system changes are in their infancy and their overall effects remain under review.
Future legislative or policy initiatives directed at reducing costs could be introduced at either the federal or state level. We cannot predict what healthcare reform legislation or regulations, if any, including any potential repeal or amendment of the ACA, will be enacted in the United States or elsewhere, what impact any legislation or regulations related to the healthcare system that may be enacted or adopted in the future might have on our business, or the effect of ongoing uncertainty or public perception about these matters will have on the purchasing decisions of our customers. However, the implementation of new legislation and regulation may materially lower reimbursements for our products, materially reduce medical procedure volumes and significantly and adversely affect our business.
If third-party payors do not provide sufficient coverage and reimbursement to healthcare providers or if there is a reduction in the number of patients with health insurance, demand for our products and our revenue could be materially and adversely affected.
Our customers rely significantly on reimbursement from public and private third-party payors procedures utilizing our radiation oncology and other medical products. Our ability to commercialize our products successfully and increase market acceptance of our products will depend in significant part on the extent to which public and private third-party payors provide adequate coverage and reimbursement for procedures that are performed with our products and the extent to which patients that are treated by our products continue to be covered by health insurance. Third-party payors may establish or change the reimbursement for medical products and services that could significantly influence the purchase of medical products and services. In addition, actions by the government, downturns in the economy and other factors outside of our control could negatively affect the number of individuals covered by health insurance. For example, in connection with COVID-19-related layoffs, many individuals have lost their employer-covered health insurance and there is uncertainty as to when or if such coverage will be re-established. If reimbursement policies or other cost containment measures are instituted in a manner that significantly reduces the coverage or payment for the procedures that are performed with our products or if there is a prolonged reduction in the number of patients eligible to be treated by our products that are covered
by health insurance, our revenue may decline, our existing customers may not continue using our products or may decrease their use of our products, and we may have difficulty obtaining new customers. Such actions would likely materially and adversely affect our business, results of operations and financial condition.
In addition, the CMS reviews reimbursement rates annually and may implement significant changes in future years, which could discourage existing and potential customers from purchasing or using our products. Further, outside of the United States, reimbursement practices vary significantly by country. Market acceptance of our products may depend on the availability and level of coverage and reimbursement in any country within a particular time.
Some of our products depend on our ability to source data from third parties who could take steps to block our access to such data. Such blocking could limit the effectiveness of these products, increase our expenses or materially and adversely impact our business.
Our SunCHECK software requires access to data such as electronic health information (“EHI”) from other third-party vendors of our customers, typically original equipment manufacturers, in order to perform quality assessments. The functioning of our analytics applications and our ability to perform analytics services is predicated on our ability to establish interfaces that download the relevant data from these third party source systems on a repeated basis and in a reliable manner. The 21st Century Cures Act, often referred to simply as the Cures Act, which was enacted in 2016, contains, among other things, incentives and penalties to promote the use and efficient exchange of EHI and prevent “information blocking” (that is, activity that is likely to interfere with, prevent, or materially discourage access, exchange, or use of EHI, where a health information technology developer, health information network or health information exchange knows or should know that a practice is likely to interfere with access to, exchange or use of EHI). While the information sharing incentives created by the Cures Act are generally beneficial to our business, the implementing regulations also contain certain exceptions which would allow a market actor to block access to EHI without liability. Consequently, we may encounter vendors that engage in information blocking practices that may inhibit our ability to access the relevant data on behalf of customers and any steps we take to enforce the anti-information blocking provisions of the 21st Century Cures Act could be costly, could distract management attention from the business, and could have uncertain results.
The impact of the 21 Century Cures Act on our business is unclear at this time, due to, among other things, uncertainty regarding the interpretation of safe harbors and exceptions to the 21 Century Cures Act by industry participants and regulators. It is unclear whether the 21 Century Cures Act may benefit us in that certain electronic health records vendors will no longer be permitted to interfere with our attempts at integration, but the rules may also make it easier for other similar companies to enter the market, creating increased competition, and reducing our market share.
Regulations related to “conflict minerals” may force us to incur additional expenses, may result in damage to our business reputation and may materially and adversely impact our ability to conduct our business.
As a public company, we will be subject to the requirements under the Dodd-Frank Wall Street Reform and Consumer Protection Act of 2010 (the “Dodd-Frank Act”) that requires us to diligence, disclose and report whether or not our devices contain conflict minerals. The implementation of these requirements could adversely affect the sourcing, availability and pricing of the materials used in the manufacture of components used in our devices. In addition, we will incur additional costs to comply with the disclosure requirements, including costs related to conducting diligence procedures to determine the sources of conflict minerals that may be used or necessary to the production of our devices and, if applicable, potential changes to devices, processes or sources of supply as a consequence of such verification activities. It is also possible that we may face reputational harm if we determine that certain of our devices contain minerals not determined to be conflict-free or if we are unable to alter our devices, processes or sources of supply to avoid such materials.
Risks Related to Our Liquidity and Capital Resources
If we cannot generate sufficient operating cash flow and obtain external financing, we may be unable to make all of our planned capital expenditures and other expenses.
Our ability to fund anticipated capital expenditures and other expenses depends on generating sufficient cash flow from operations and the availability of external financing.
Our debt service obligations and our capital expenditures, together with on-going operating expenses, are expected to be a substantial drain on our cash flow and may decrease our cash balances. The timing and amount of our capital requirements cannot be precisely determined at this moment and will depend on a number of factors, including demand for our products, product mix, changes in industry conditions and market competition. We intend to regularly assess markets for external financing opportunities, including debt and equity. Such financing may not be available when needed or, if available, may
not be available on satisfactory terms, particularly in light of the limited financing available as a result of the recent global financial crisis. Any equity financing would cause further dilution to our stockholders. Our inability to obtain needed financing or to generate sufficient cash from operations may require us to abandon projects or curtail capital expenditures, and we could be materially adversely affected. If we are not able to independently generate excess free cash flow and obtain third party debt or equity financing, our ability to grow our business may be materially and adversely affected.
On October 20, 2021, certain subsidiaries of the Company entered into a credit agreement (the “Credit Agreement”) among Mirion Technologies (HoldingSub2), Ltd., a limited liability company incorporated in England and Wales, as Holdings, Mirion Technologies (US Holdings), Inc., as the Parent Borrower, Mirion Technologies (US), Inc., as the Subsidiary Borrower, the lending institutions party thereto, Citibank, N.A., as the Administrative Agent and Collateral Agent and Goldman Sachs Lending Partners, Citigroup Global Markets Inc., Jefferies Finance LLC and JPMorgan Chase Bank, N.A., as the Joint Lead Arrangers and Bookrunners. The Credit Agreement provides for an $830 million senior secured first lien term loan facility (the “Term Facility”) and a $90 million senior secured revolving facility (the “Revolving Facility” and, together with the Term Facility, the “Credit Facilities”).
Our indebtedness may have important consequences, including, but not limited to, the following:
•increasing our vulnerability to general economic downturns and adverse industry conditions;
•requiring us to dedicate a significant portion of our cash flows from operations to the payment of interest and principal on our debt, which would reduce the funds available to us for our working capital, capital expenditures or other general corporate requirements;
•limiting our flexibility in planning for, or reacting to, changes in our business and industry;
•placing us at a competitive disadvantage compared to our competitors with less indebtedness or more liquidity;
•limiting our ability to obtain additional financing in the future for working capital, capital expenditures, acquisitions, general corporate purposes or other purposes; and
•exposure to market conditions impact on our variable interest rate debt.
For more information, see “Part II, Item 7. Management’s Discussion and Analysis of Financial Condition and Results of Operations—Liquidity and Capital Resources—Debt Profile.”
Despite our levels of indebtedness, we have the ability to incur more indebtedness. Incurring additional debt could further intensify the risks described above.
We may incur additional debt in the future and the terms of the Credit Agreement permit us to do so subject to certain limitations. We have the ability to draw upon our $90 million Revolving Facility. We also have the ability to utilize the uncommitted “accordion” under the Credit Facilities (subject to the receipt of commitments and satisfaction of certain other conditions), which permits the incurrence of additional debt if certain incurrence and leverage ratio tests in the Credit Agreement are satisfied, and the Credit Agreement contains other provisions allowing us to incur significant amounts of additional debt. If additional debt is added to the debt that is originally incurred under the Credit Facilities, the related risks could intensify and we may not be able to meet all our respective debt obligations. In addition, the Credit Agreement does not prevent us from incurring obligations that do not constitute indebtedness as defined therein.
Restrictive covenants in the Credit Agreement and any future debt agreements, could restrict our operating flexibility.
The Credit Agreement contains restrictive covenants that limit our ability to engage in specified transactions and prohibit us from voluntarily prepaying certain of our other indebtedness. These covenants limit our ability to, among other things:
•incur additional indebtedness;
•pay dividends on, or repurchase or make distributions in respect of, our capital stock or make other restricted payments;
•make certain investments, including acquisitions of other companies;
•sell or transfer assets;
•prepay, redeem, repurchase, defease or amend the terms of certain junior indebtedness;
•create or incur liens on our assets or enter into contractual obligations that restrict our ability to grant liens on assets or capital stock; and
•consolidate, merge, sell or otherwise dispose of all or substantially all of our assets.
Under the Credit Agreement, in certain circumstances we also are required to satisfy and maintain a certain “First Lien Net Leverage Ratio” (as defined in the Credit Agreement). Our ability to meet this financial ratio could be affected by events beyond our control, and there can be no assurance that we will meet that ratio.
The failure to comply with any of these covenants or any other term of the Credit Agreement could cause a default under the Credit Agreement. A default, if not waived, could result in acceleration of the outstanding indebtedness under the Credit Agreement, in which case such indebtedness would become immediately due and payable, and could also cause the acceleration of other indebtedness outstanding at such time. If any default occurs, we may not be able to pay our debt or borrow sufficient funds to refinance it. Even if new financing is available, it may not be available on terms that are acceptable to us. Complying with these covenants may cause us to take actions that we otherwise would not take or not take actions that we otherwise would take.
The expected replacement of the LIBOR benchmark interest rate and other interbank offered rates with new benchmark rate indices may have an impact on our financing costs.
LIBOR, the interest rate benchmark used as a reference rate on our variable rate debt, including our Credit Agreement is being phased out. As of December 31, 2021, we had approximately $828 million of debt outstanding under the Credit Agreement with interest rates based on LIBOR. The Credit Agreement includes fallback language that seeks to either facilitate an agreement with our lenders on a replacement rate for LIBOR in the event of its discontinuance or that automatically replaces LIBOR with benchmark rates based on the Secured Overnight Financing Rate ("SOFR") or other benchmark replacement rates upon certain triggering events. There are many uncertainties regarding a transition from LIBOR and we cannot predict what the impact of any such replacement rate would be to our interest expense. The discontinuation, reform, or replacement of LIBOR or any other benchmark rates may result in the need to amend all contracts with LIBOR or such other benchmark rates, and this may have a negative impact on our interest expense and our profitability. The consequences of these developments with respect to LIBOR cannot be entirely predicted and span multiple future periods. Potential changes to the underlying floating-rate indices and reference rates may have an adverse impact on our liabilities indexed to LIBOR and could have a negative impact on our profitability and cash flows. Furthermore, we cannot predict or quantify the time, effort and cost required to transition to the use of SOFR or new benchmark rates, including with respect to negotiating and implementing any necessary changes to existing contractual agreements, and implementing changes to our systems and processes. We continue to evaluate the operational and other effects of such changes, including possible impacts on our accounting for interest rate hedging agreements.
Unfavorable currency exchange rate fluctuations could materially and adversely affect our financial results.
Our international sales and our operations in countries other than the United States expose us to risks associated with fluctuating currency values and exchange rates. A significant amount of our international sales, costs, assets and liabilities are denominated in currencies other than the U.S. dollar. For example, in fiscal 2021, approximately 39% of our sales were denominated in euros, 3% in pounds sterling, 2% in Japanese yen and 2% in Canadian dollars. Gains and losses on the conversion of accounts receivable, accounts payable and other monetary assets and liabilities to U.S. dollars may contribute to fluctuations in our results of operations. In addition, increases in the value of the U.S. dollar relative to the euro could have an adverse effect on our results of operations. We do not currently purchase forward contracts to hedge against the risks associated with fluctuations in exchange rates.
Changes in our effective tax rate, including as a result of changes in law or recent changes in our organizational structure occurring, or adverse outcomes resulting from examination of our income tax returns, could materially and adversely affect our results of operations.
Our effective tax rate could be adversely affected by several factors, many of which are outside of our control, including:
•earnings being lower than anticipated in countries where we are taxed at lower rates or other shifts in the mix of pre-tax profits and losses from one jurisdiction to another;
•our inability to use tax credits;
•changing tax laws or related interpretations, accounting standards and regulations and interpretations in multiple tax jurisdictions in which we operate;
•an increase in expenses not deductible for tax purposes, including certain share-based compensation expense and impairment of goodwill;
•the tax effects of purchase accounting for acquisitions and restructuring charges and other discrete recognition of taxable events and exposures that may cause fluctuations between reporting periods;
•changes related to our ability to ultimately realize future benefits attributed to net operating loss and other carryforwards included in our deferred tax assets;
•tax assessments resulting from income tax audits or any related tax interest or penalties that would affect our income tax expense for the period in which the settlements take place; and
•a change in our decision to indefinitely reinvest foreign earnings.
For example, on October 28, 2021, the Biden administration proposed changes to the U.S. tax system. The proposals under discussion include changes to the U.S. corporate tax system that would impose a corporate minimum book tax and increase the tax rate on and make other tax changes to GILTI earned by foreign subsidiaries. Many aspects of the current proposals are unclear or undeveloped, and we are unable to predict which, if any, U.S. tax reform proposals will be enacted into law, and what effects any enacted legislation might have on our liability for U.S. federal income taxes. However, it is possible that the enactment of changes in the U.S. corporate tax system could materially and adversely affect our liability for U.S. corporate tax and our consolidated effective tax rate.
Changes in our organizational structure occurring in connection with the Business Combination may also impact our tax rate. For example, prior to the Business Combination, income derived by many of our non-U.S. subsidiaries was not subject to U.S. federal income tax but, after the Business Combination, we will be subject to U.S. federal income tax on our worldwide income, including in certain cases dividends from, or income earned by, our non-U.S. subsidiaries, which may adversely impact our overall effective tax rate. In addition, we expect to have significantly reduced non-deductible interest expense in periods following the Business Combination, which may impact our effective tax rate. As a result, we can provide no assurances as to how our effective tax rate is expected to be impacted by our post-Business Combination organizational structure. If our effective tax rate were to increase, our business, results of operations and financial condition could be materially and adversely affected.
In addition, we may be subject to examination of our income tax returns by the U.S. Internal Revenue Service or other tax authorities. If any tax authority challenges the relative mix of our U.S. and international income, our future effective income tax rates could be adversely affected. While we regularly assess the likelihood of adverse outcomes from such examinations and the adequacy of our provision for income taxes, we cannot assure you that such provision is sufficient and that a determination by a tax authority will not have an adverse effect on our business, results of operations and financial condition.
Risks Related to Ownership of our Securities
The price of our Class A common stock and warrants may be volatile.
The price of our Class A common stock and our warrants may fluctuate due to a variety of factors, including:
•changes in the industries in which we and our customers operate;
•developments involving our competitors;
•changes in laws and regulations affecting our business;
•variations in our operating performance and the performance of our competitors in general;
•actual or anticipated fluctuations in our quarterly or annual operating results;
•publication of research reports by securities analysts about us or our competitors or our industry;
•the public’s reaction to our press releases, our other public announcements and our filings with the SEC;
•actions by stockholders, including the sale by the PIPE Investors of any of their shares of our Class A common stock;
•the potential sales of 18,750,000 founder shares upon the satisfaction of certain vesting requirements;
•the issuance and potential sales of 8,560,540 shares of Class A common stock upon the redemption of shares of IntermediateCo Class B common stock;
•the issuance and potential sales of 27,249,979 shares of Class A common stock upon the exercise of the public warrants and private placement warrants;
•the sales of shares of our common stock after the expiration of applicable lockup restrictions;
•additions and departures of key personnel;
•commencement of, or involvement in, litigation involving the combined company;
•changes in our capital structure, such as future issuances of securities or the incurrence of additional debt;
•the volume of shares of our Class A common stock available for public sale; and
•general economic and political conditions, such as the effects of the COVID-19 outbreak, recessions, interest rates, local and national elections, fuel prices, international currency fluctuations, corruption, political instability and acts of war or terrorism.
In addition, the stock market in general has experienced extreme price and volume fluctuations that have often been unrelated or disproportionate to the operating performance of listed companies. Broad market and industry factors may significantly impact the market price of our Class A common stock and warrants, regardless of our actual operating performance. In addition, in the past, following periods of volatility in the overall market and the market prices of a particular company’s securities, securities class action litigation has often been instituted against that company. Securities litigation, if instituted against us, could result in substantial costs and divert our management’s attention and resources from our business. Any of the factors listed above could materially and adversely affect your investment in our securities, and our securities may trade at prices significantly below the price you paid for them. In such circumstances, the trading price of our securities may not recover and may experience a further decline.
The coverage of our business or our securities by securities or industry analysts or the absence thereof could adversely affect the price of our securities and trading volume.
The trading market for our securities will be influenced in part by the research and other reports that industry or securities analysts may publish about us or our business or industry from time to time. We do not control these analysts or the content and opinions included in their reports. As a former special purpose acquisition company, we may be slow to attract equity research coverage, and the analysts who publish information about our securities will have had relatively little experience with our company, which could affect their ability to accurately forecast our results and make it more likely that we fail to meet their estimates. If no or few analysts commence equity research coverage of us, the trading price and volume of our securities would likely be negatively impacted. If analysts do cover us and one or more of them downgrade our securities, or if they issue other unfavorable commentary about us or our industry or inaccurate research, our stock price would likely decline. Furthermore, if one or more of these analysts cease coverage or fail to regularly publish reports on us, we could lose visibility in the financial markets. Any of the foregoing would likely cause our stock price and trading volume to decline.
Even if we are actively covered by analysts, we do not have any control over the analysts or the measures that analysts or investors may rely upon to forecast our future results. Overreliance by analysts or investors on any particular metric to forecast our future results may lead to forecasts that differ significantly from our own.
We may require additional capital to support our growth plans, and such capital may not be available on terms acceptable to us, if at all. This could hamper our growth and adversely affect our business.
We intend to continue to make significant investments to support our business growth and may require additional funds to respond to business challenges, improve our operating infrastructure or acquire complementary businesses, personnel and technologies. Accordingly, we may need to engage in equity or debt financings to secure additional funds, including for possible use in acquisitions. If we raise additional funds through future issuances of equity or convertible debt securities, our existing stockholders could suffer significant dilution, and any new equity securities we issue could have rights, preferences and privileges superior to those of holders of our Class A common stock.
Any additional debt financing that we secure in the future could involve offering additional security interests and undertaking restrictive covenants relating to our capital raising activities and other financial and operational matters, which may make it more difficult for us to obtain additional capital and to pursue business opportunities, including potential acquisitions. Additionally, the COVID-19 pandemic has disrupted capital markets, and if we seek to access additional capital or increase our borrowing, there can be no assurance that debt or equity financing may be available to us on favorable terms, if at all. If we are unable to obtain adequate financing or financing on terms satisfactory to us when we require it, our ability to continue to support our business growth and to respond to business challenges could be significantly impaired, and our business, results of operations and financial condition may be harmed.
Our warrants are exercisable for our Class A common stock, we may elect to issue shares of our Class A common stock in connection with the redemption of shares of IntermediateCo Class B common stock and the founder shares may vest, each of which would increase the number of shares eligible for future resale in the public market and result in dilution to our stockholders.
Outstanding warrants to purchase an aggregate of 27,249,979 shares of our Class A common stock (including 18,749,979 public warrants and 8,500,000 private placement warrants) are exercisable. The exercise price of these warrants is $11.50 per share. In addition, up to 8,560,540 shares of Class A common stock may be issued in connection with the redemption of IntermediateCo Class B common stock and up to 18,750,000 founder shares may vest and become unrestricted upon the occurrence of certain vesting requirements. To the extent such warrants are exercised and such shares are issued or become unrestricted, additional shares of our Class A common stock will be issued or become eligible for resale, which will result in dilution to the holders of our common stock and increase the number of shares eligible for resale in the public market. Sales of substantial numbers of such shares in the public market or the fact that such warrants may be exercised could adversely affect the market price of our Class A common stock.
The public warrants may never be in the money, and they may expire worthless and the terms of the warrants may be amended in a manner adverse to a holder if holders of at least 50% of the then outstanding public warrants approve of such amendment.
The warrants were issued in registered form under a warrant agreement with Continental Stock Transfer & Trust Company, N.A., as warrant agent, and us. The warrant agreement provides that the terms of the warrants may be amended without the consent of any holder to cure any ambiguity or correct any defective provision, but requires the approval by the holders of at least 50% of the then outstanding public warrants to make any change that adversely affects the interests of the registered holders of public warrants. Accordingly, we may amend the terms of the public warrants in a manner adverse to a holder if holders of at least 50% of the then outstanding public warrants approve of such amendment. Although our ability to amend the terms of the public warrants with the consent of at least 50% of the then outstanding public warrants is unlimited, examples of such amendments could be amendments to, among other things, increase the exercise price of the warrants, convert the warrants into cash or stock (at a ratio different than initially provided), shorten the exercise period or decrease the number of shares of our Class A common stock purchasable upon exercise of a warrant.
We may redeem your unexpired warrants prior to their exercise at a time that is disadvantageous to you, thereby making your warrants worthless.
We have the ability to redeem outstanding warrants, in whole and not in part, at any time prior to their expiration, at a price of $0.01 per warrant, provided that the last reported sales price of our Class A common stock equals or exceeds $18.00 per share (as adjusted for stock splits, stock dividends, reorganizations, recapitalizations and the like) for any 20 trading days within a 30 trading-day period ending on the third trading day prior to the date we send the notice of redemption to the
warrant holders. If and when the warrants become redeemable by us, we may exercise our redemption right even if we are unable to register or qualify the underlying securities for sale under all applicable state securities laws.
In addition, we may redeem the outstanding warrants, in whole and not in part at a price of $0.10 per warrant provided that:
•holders will be able to exercise their warrants on a cashless basis prior to redemption and receive that number of shares of Class A common stock provided for in the warrant agreement;
•if, and only if, the last reported sale price of our Class A common stock equals or exceeds $10.00 per share (as adjusted for stock splits, stock dividends, reorganizations, recapitalizations and the like) on the trading day prior to the date on which we send the notice of redemption to the warrant holders; and
•if, and only if, there is an effective registration statement covering the issuance of the shares of Class A common stock issuable upon exercise of the warrants and a current prospectus relating thereto available throughout the 30-day period after written notice of redemption is given.
Such redemption may occur at a time when the warrants are “out-of-the-money,” in which case you would lose any potential embedded value from a subsequent increase in the value of the Class A common stock had your warrants remained outstanding.
Redemption of the outstanding warrants could force you to: (1) exercise your warrants and pay the exercise price therefor at a time when it may be disadvantageous for you to do so; (2) sell your warrants at the then-current market price when you might otherwise wish to hold your warrants; or (3) accept the nominal redemption price which, at the time the outstanding warrants are called for redemption, is likely to be substantially less than the market value of your warrants.
None of the private placement warrants will be redeemable by us so long as they are held by the Sponsor or its permitted transferees.
Our warrants are accounted for as derivative liabilities and the changes in the value of our warrants have had and may continue to have a material effect on our financial results.
Our warrants are included on our balance sheet as of December 31, 2021 as derivative liabilities. ASC 815 provides for the remeasurement of the fair value of such derivatives at each balance sheet date, with a resulting non-cash gain or loss related to the change in the fair value being recognized in earnings in the statement of operations. As a result of the recurring fair value measurement, our financial statements and results of operations have fluctuated and may continue to fluctuate quarterly, based on factors which are outside of our control. Due to the recurring fair value measurement, we expect that we will recognize non-cash gains or losses on our warrants each reporting period and that the amount of such gains or losses could be material.
There is no guarantee that our warrants will be in the money, and they may expire worthless and the terms of our warrants may be amended.
The exercise price for our warrants is $11.50 per share of Class A common stock. There is no guarantee that the warrants will be in the money at any given time prior to their expiration on October 20, 2026. If the trading price of our common stock declines, the warrants may expire worthless.
We have not and may not pay cash dividends for the foreseeable future.
We currently intend to retain our future earnings, if any, to finance the further development and expansion of our business and does not intend to pay cash dividends in the foreseeable future. Any future determination to pay dividends will be at the discretion of our Board and will depend on our financial condition, results of operations, capital requirements, restrictions contained in future agreements and financing instruments, business prospects and such other factors as our Board deems relevant.
We will have broad discretion over the use of proceeds from the exercise of the warrants, and we may invest or spend the proceeds in ways with which investors do not agree and in ways that may not yield a return.
We will have broad discretion over the use of proceeds from the exercise of warrants. Investors may not agree with our decisions, and our use of the proceeds may not yield a return on investment. We intend to use these net proceeds for general corporate purposes, which may include capital expenditures, investments and working capital. In addition, from time to time in the past we have considered, and we continue to consider, acquisitions and strategic transactions, and we
also may use such net proceeds for such purposes. Our use of these proceeds may differ substantially from our current plans. Our failure to apply the net proceeds from the exercises of warrants and options effectively could impair our ability to pursue our growth strategy or could require us to raise additional capital.
We are subject to certain ownership and voting power laws and regulations which may limit the ability of stockholders to acquire our Class A common stock and therefore limit demand for our Class A common stock.
Under foreign direct investment and public interest laws, including in Germany, Finland, France, and the UK, and potentially other jurisdictions, certain acquisitions of our Class A common stock by investors are subject to government approval requirements. For example, in Germany, German foreign direct investment law require foreign investors to obtain approval from the German Federal Ministry for Economic Affairs and Energy for the direct or indirect acquisition of shares of a German company if the acquirer directly or indirectly holds at least 10% of the voting rights of the company following the acquisition. Any acquisition in violation of the aforementioned provisions of German foreign direct investment law may be void. Any violation of the prohibition to consummate an acquisition without approval of the Ministry may be subject to sanctions. Similar foreign direct investment laws exist in other jurisdictions in which we have substantial operations. In Finland, government approvals are required if an investor holds at least 10% of the voting rights of the company following the investment. In France, the prior approval from the French Minister of Economy is required if a non-EU investor exceeds, directly or indirectly, 25% of the voting rights of the French entities of the company following the investment or, for an EU non-French investor, in case of acquisition of control, direct or indirect, of the French entities. The U.K. has a 25% voting rights threshold for mandatory filings under the National Security and Investment Act 2021 which became operational on January 4, 2022. Accordingly, these restrictions on and approval requirements for the acquisition of a substantial shareholding in our share capital may restrict certain investments and limit demand for shares of our Class A common stock.
Anti-takeover provisions contained in our Charter and Bylaws, as well as provisions of Delaware law, could impair a takeover attempt.
Our Charter and Bylaws contain provisions that may discourage unsolicited takeover proposals that stockholders may consider to be in their best interests. We are also subject to anti-takeover provisions under Delaware law, which could delay or prevent a change of control. Together, these provisions may make more difficult the removal of management and may discourage transactions that otherwise could involve payment of a premium over prevailing market prices for our securities. Certain of these provisions provide:
•no cumulative voting in the election of directors, which limits the ability of minority stockholders to elect director candidates;
•the right of our Board to elect a director to fill a vacancy created by the expansion of our Board or the resignation, death or removal of a director in certain circumstances, which prevents stockholders from being able to fill vacancies on our Board;
•a prohibition on stockholder action by written consent, which forces stockholder action to be taken at an annual or special meeting of our stockholders;
•a prohibition on stockholders calling a special meeting and the requirement that a meeting of stockholders may only be called by members of our Board or our Chief Executive Officer, which may delay the ability of our stockholders to force consideration of a proposal or to take action, including the removal of directors; and
•advance notice procedures that stockholders must comply with in order to nominate candidates to our Board or to propose matters to be acted upon at a meeting of stockholders, which may discourage or deter a potential acquirer from conducting a solicitation of proxies to elect the acquirer’s own slate of directors or otherwise attempting to obtain control of us.
Our Charter includes forum selection clauses, which could discourage claims or limit stockholders’ ability to make a claim against us, our directors, officers, other employees or stockholders.
Our Charter provides that, unless we consent in writing to the selection of an alternative forum, the Court of Chancery in the State of Delaware shall be the sole and exclusive forum for any stockholder (including a beneficial owner) to bring: (a) any derivative action or proceeding brought on behalf of the Company; (b) any claim or cause of action for breach of a fiduciary duty owed by any current or former director, officer or other employee of the Company, to the Company or the Company’s stockholders; (c) any claim or cause of action against the Company or any current or former director, officer or other employee of the Company, arising out of or pursuant to any provision of the DGCL or our certificate of incorporation
or bylaws; (d) any claim or cause of action seeking to interpret, apply, enforce or determine the validity of our certificate of incorporation or bylaws (as each may be amended from time to time, including any right, obligation, or remedy thereunder), (e) any claim or cause of action as to which the DGCL confers jurisdiction on the Court of Chancery of the State of Delaware; and (f) any claim or cause of action against the Company or any current or former director, officer or other employee of the Company, governed by the internal- affairs doctrine, in all cases to the fullest extent permitted by law and subject to the court having personal jurisdiction over the indispensable parties named as defendants. In addition, our Charter provides that, unless we consent in writing to the selection of an alternative forum, the federal district courts of the United States will be the exclusive forum for resolving any complaint asserting a cause of action arising under the Securities Act.
Notwithstanding the foregoing, the Securities Act forum selection clause will not apply to suits brought to enforce any liability or duty created by the Exchange Act or any other claim for which the federal district courts of the United States of America shall be the sole and exclusive forum. These forum selection clauses may discourage claims or limit stockholders’ ability to submit claims in a judicial forum that they find favorable and may result in additional costs for a stockholder seeking to bring a claim. While we believe the risk of a court declining to enforce these forum selection clauses is low, if a court were to determine a forum selection clause to be inapplicable or unenforceable in an action, we may incur additional costs in conjunction with our efforts to resolve the dispute in an alternative jurisdiction, which could have a negative impact on our business, results of operations and financial condition.
We may be subject to securities litigation, which is expensive and could divert management attention and result in significant legal expenses and settlement or damage awards.
The market price of our Class A common stock may be volatile and, in the past, companies that have experienced volatility in the market price of their stock have been subject to securities class action litigation. We have and may in the future become subject to claims and litigation alleging violations of the securities laws or other related claims, which could harm our business and require us to incur significant costs. We are generally obliged, to the extent permitted by law, to indemnify our current and former directors and officers who are named as defendants in these types of lawsuits. Regardless of the outcome, litigation may require significant attention from management and could result in significant legal expenses, settlement costs or damage awards that could materially and adversely affect our business, results of operations and financial condition.
ITEM 1B. UNRESOLVED STAFF COMMENTS
None.
ITEM 2. PROPERTIES
The Company's principal executive offices are located at 1218 Menlo Drive, Atlanta, Georgia. Our headquarters facilities consist of two buildings, which we have leased through 2031. The buildings contain approximately 31,000 square feet of floor space. The Company also leases administrative offices, as well as engineering, production and warehouse space in various locations in the United States, Canada, France, Germany, the United Kingdom, Finland, Estonia, The Netherlands, China, Japan, and South Korea. In addition to these leased properties, we also own facilities in Belgium, France, Canada and the United States. We believe that these facilities are suitable and adequate to meet our current operating needs.
ITEM 3. LEGAL PROCEEDINGS
Due to the nature of our activities, we are at times subject to pending and threatened legal actions that arise out of the ordinary course of business. For information regarding legal proceedings and other claims in which we are involved, see “Note 10. Commitments and Contingencies” in the notes to the financial statements included in this Annual Report on Form 10-K. The disposition of any such currently pending or threatened matters is not expected to have a material effect on our business, results of operations or financial condition. However, the results of legal actions cannot be predicted with certainty. Therefore, it is possible that our business, results of operations and financial condition could be materially adversely affected in any particular period by the unfavorable resolution of one or more legal actions. Regardless of the outcome, litigation can have an adverse impact on our business because of defense and settlement costs, diversion of management resources and other factors. In addition, the expense of litigation and the timing of this expense from period to period are difficult to estimate, subject to change and could adversely affect our consolidated financial statements.
ITEM 4. MINE SAFETY DISCLOSURES
Not applicable.
PART II
ITEM 5. MARKET FOR REGISTRANT’S COMMON EQUITY, RELATED STOCKHOLDER MATTERS AND ISSUER PURCHASES OF EQUITY SECURITIES
Market Information
Prior to the consummation of the Business Combination, our publicly-traded Class A common stock, units and warrants were listed on the NYSE under the symbols “GSAH,” “GSAH.U” and “GSAH WS,” respectively. Since the consummation of the Business Combination, our Class A common stock and warrants are listed on the NYSE under the symbols “MIR” and “MIR WS,” respectively. Since the consummation of the Business Combination, our outstanding units that were not previously separated into the underlying shares of Class A common stock and one-fourth of a warrant were cancelled and each unit holder received one share of Class A common stock and one-fourth of a warrant, provided that no fractional warrants were issued upon separation of our units. Such units no longer trade as a separate security and were delisted from the NYSE.
Holders
As of February 22, 2022, the company had 199,523,292 shares of Class A common stock, including 18,750,000 founder shares subject to vesting requirements, outstanding held of record by approximately 75 holders, 8,560,540 shares of Class B common stock outstanding held of record by approximately 17 holders, outstanding warrants to purchase 27,249,979 shares of Class A common stock held of record by approximately 2 holders and no shares of preferred stock outstanding. Such amounts do not include DTC participants or beneficial owners holding shares through nominee names.
Dividends
We have not paid any cash dividends on common stock to date. Our ability to pay dividends is limited by restrictions on our ability to pay dividends or make distributions under the terms of the Credit Facilities. Any future determination to declare dividends will be made at the discretion of our board of directors, subject to applicable laws, and will depend on a number of factors, including our financial condition, results of operations, capital requirements, contractual restrictions, general business conditions and other factors that our board of directors may deem relevant.
Securities Authorized for Issuance under Equity Compensation Plans
The following table provides information as of December 31, 2021 with respect to our shares of Class A common stock issuable under our equity compensation plans.
Equity Compensation Plan Information
| | | | | | | | | | | |
| Plan Category | Number of Securities to be issued upon exercise of outstanding options, warrants and rights | Weighted-average exercise price of outstanding options, warrants and rights | Number of securities remaining available for issuance under equity compensation plans (excluding securities reflected in column (a)) |
Equity compensation plans approved by security holders(1) | 1,238,683 (2) | — | | 18,713,646 (3) |
| Equity compensation plans not approved by security holders | — | — | — |
| Total | 1,238,683 | | — | 18,713,646 | |
(1) Includes the 2021 Omnibus Incentive Plan (the “Omnibus Plan”).
(2) Represents 1,238,683 shares of Class A common stock subject to restricted stock units (“RSUs”) and performance stock units (“PSUs”) outstanding as of December 31, 2021. RSUs and PSUs do not have an exercise price.
(3) As of December 31, 2021, an aggregate of 18,713,646 shares of Class A common stock were available for issuance under the Omnibus Plan. The number of shares of Class A common stock reserved for issuance under the Omnibus Plan will automatically increase on the first day of each fiscal year by the lesser of (i) 3% of the total number of outstanding shares of Class A common stock on the last day of the immediately preceding fiscal year, (ii) 9,976,164 shares of the Class
A common stock and (iii) such smaller number of shares of Class A common stock as determined by the compensation committee of our board of directors.
Performance Graph
The graph below compares the cumulative total return for our shares of Class A common stock from August 20, 2020 through December 31, 2021 with the comparable cumulative return of three indices: the S&P 500 Index (“S&P 500”), Nasdaq and the Dow Jones Industrial Average Index (“DJIA”). The graph assumes $100 invested on August 20, 2020 in each of our Class A common stock and the three indices presented. The stock price performance included in the below graph is not necessarily indicative of future stock performance. The Business Combination closed on October 20, 2021 and GSAH was renamed Mirion Technologies, Inc. and, pursuant to the terms of the Business Combination Agreement, Mirion TopCo combined with a subsidiary of GSAH. Our Class A common stock is listed on the NYSE under the ticker symbol “MIR.” The graph below represents GSAH until October 20, 2021 and MIR from October 20, 2021 to December 31, 2021.
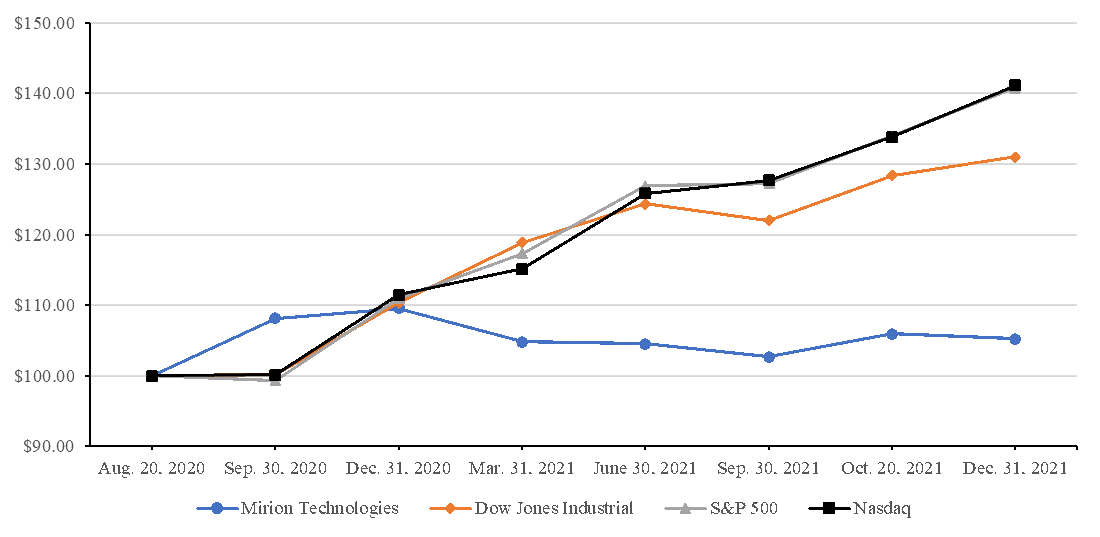
This performance graph shall not be deemed “soliciting material” or to be “filed” with the SEC for purposes of Section 18 of the Exchange Act, or otherwise subject to the liabilities under that Section, and shall not be deemed to be incorporated by reference into any our filings under the Securities Act or the Exchange Act.
Recent Sales of Unregistered Securities; Use of Proceeds from Registered Offerings
The information required has been previously disclosed in our Current Report on Form 8-K filed with the Securities and Exchange Commission on October 25, 2021.
Issuer Purchases of Equity Securities
None.
ITEM 7. MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS
You should read the following discussion and analysis of Mirion’s financial condition and results of operations together with the consolidated financial statements and related notes of Mirion Technologies, Inc. that are included elsewhere in this Annual Report on Form 10-K. This discussion contains forward-looking statements based upon current expectations that involve risks and uncertainties. Our actual results may differ materially from those anticipated in these forward-looking statements as a result of various factors, including those set forth under the section entitled “Part I, Item 1A. Risk Factors” or in other parts of this Annual Report on Form 10-K. Please also see the section entitled “Cautionary Note Regarding Forward-Looking Statements.” Unless the context otherwise requires, references in this section to “we,” “us,” “our,” “Mirion” and “the Company” refer to the business and operations of Mirion Technologies TopCo, Ltd. and its consolidated subsidiaries prior to the Business Combination and to Mirion and its consolidated subsidiaries, following the consummation of the Business Combination. Unless the context otherwise requires or unless otherwise specified, all dollar amounts in this section are in millions.
Overview
We are a global provider of products, services, and software that allow our customers to safely leverage the power of ionizing radiation for the greater good of humanity through critical applications in the medical, nuclear and defense markets, as well as laboratories, scientific research, analysis, and exploration.
We provide dosimetry solutions for monitoring the total amount of radiation medical staff members are exposed to over time, radiation therapy quality assurance solutions for calibrating and verifying imaging and treatment accuracy, and radionuclide therapy products for nuclear medicine applications such as shielding, product handling, medical imaging furniture, and rehabilitation products. We provide robust, field-ready personal radiation detection and identification equipment for defense applications and radiation detection and analysis tools for power plants, labs, and research applications. Nuclear power plant product offerings are used for the full nuclear power plant lifecycle including core detectors, essential measurement devices for new build, maintenance, decontamination and decommission, and equipment for monitoring and control during fuel dismantling and remote environmental monitoring.
We manage and report results of operations in two business segments: Medical and Industrial.
•Our revenues were $154.1 million for the Successor Period and $168.0 million for the Predecessor Stub Period, of which 31.9% and 35.9% was generated in the Medical segment for the Successor and Predecessor Stub Periods, respectively, and 68.1% and 64.1% was generated in the Industrial segment for the Successor and Predecessor Periods, respectively. Revenues for the six months ended December 31, 2020 were $265.4 million, with 19.6% and 80.4% generated in the Medical and Industrial segments, respectively.
•Backlog (representing committed but undelivered contracts and purchase orders, including funded and unfunded government contracts) was $747.5 million and $715.8 million as of December 31, 2021, and June 30, 2021, respectively.
The Mirion Business Combination
The Business Combination closed on October 20, 2021 (the “Closing Date”), and GSAH was renamed Mirion Technologies, Inc. Our Class A common stock is listed on the NYSE under the ticker symbol “MIR.”
The Business Combination is being accounted for under ASC 805, Business Combinations. GSAH has been determined to be the accounting acquirer. Mirion constitutes a business in accordance with ASC 805 and the Business Combination constitutes a change in control. Accordingly, the Business Combination is being accounted for using the acquisition method. Under this method of accounting, Mirion is treated as the “acquired” company for financial reporting purposes and our net assets are stated at fair value, with goodwill or other intangible assets recorded.
On October 20, 2021, the Board of Directors determined to change Mirion TopCo's fiscal year end from June 30 of each year to December 31 of each year. The determination was made to align Mirion’s fiscal year end with GSAH’s fiscal year end. See the "Basis of Presentation" section below for further details regarding the impact of the Business Combination and the change in fiscal year-end on the presentation of our financial statements.
As a result of the Business Combination, Mirion’s financial statement presentation distinguishes Mirion TopCo as the “Predecessor” for periods prior to the closing of the Business Combination and Mirion Technologies, Inc. as the “Successor” for periods after the closing of the Business Combination. As a result of the application of the acquisition method of accounting in the Successor Period, the financial statements for the Successor Period are presented on a full
step-up basis as a result of the Business Combination, and are therefore not comparable to the financial statements of the Predecessor Period that are not presented on the same full step-up basis due to the Business Combination.
Key Factors Affecting Our Performance
We believe that our business and results of operations and financial condition may be impacted in the future by various trends and conditions, including the following:
•Global risk—Our business depends in part on operations and sales outside the United States. Risks related to those international operations and sales include new foreign investment laws, new export/import regulations, and additional trade restrictions (such as sanctions and embargoes). New laws that favor local competitors could prevent our ability to compete outside the United States. Additional potential issues are associated with the impact of these same risks on our suppliers and customers. If our customers or suppliers are impacted by these risk factors, we may see the reduction or cancellation of customer orders, or interruptions in raw materials and components.
•Tariffs or Sanctions—The United States imposes tariffs on imports from China and other countries, which has resulted in retaliatory tariffs and restrictions implemented by China and other countries. There are, at any given time, a multitude of ongoing or threatened armed conflicts around the world. As one example, United States and other countries sanctions against Russian entities or individuals related to developments in Ukraine, along with any Russian retaliatory measures could increase our costs, adversely affect our operations, or impact our ability to meet existing contractual obligations.
•Medical end market trends—Growth and operating results in our Medical segment are impacted by:
•Increased or changes to global regulatory standards;
•Increased focus on healthcare safety;
•Changes to healthcare reimbursement;
•Potential budget constraints in hospitals and other healthcare providers;
•Medical/lab dosimetry growth supported by growing and aging demographics, increased number of healthcare professionals, and penetration of radiation therapy/diagnostics; and
•Medical radiation therapy quality assurance (“RT QA”) growth driven by growing and aging population demographics, low penetration of RT QA technology in emerging markets, and increased adoption of advanced software and hardware solutions for improved outcomes and administrative and labor efficiencies.
•Business combinations—A large driver of our historical growth has been the acquisition and integration of related businesses. Our ability to integrate, restructure, and leverage synergies of these businesses will impact our operating results over time.
•Environmental objectives of governments—Growth and operating results in our Industrial segment are impacted by environmental policy decisions made by governments in the countries where we operate. Our nuclear power customers may benefit from decarbonization efforts given the relatively low carbon footprint of nuclear power to other existing energy sources. In addition, decisions by governments to build new power plants or decommission existing plants can positively and negatively impact our customer base.
•Government budgets—While we believe that we are poised for growth from governmental customers in both of our segments, our revenues and cash flows from government customers are influenced, particularly in the short-term, by budgetary cycles. This impact can be either positive or negative.
•Nuclear new build projects—A portion of our backlog is driven by contracts associated with the construction of new nuclear power plants. These contracts can be long-term in nature and provide us with a strong pipeline for the recognition of future revenues in our Industrial segment. We perform our services and provide our products at a fixed price for certain contracts. Fixed-price contracts carry inherent risks, including risks of losses from underestimating costs, operational difficulties and other changes that may occur over the contract period. If our cost estimates for a contract are inaccurate or if we do not execute the contract within our cost estimates, we may incur losses or the contract may not be as profitable as we expected. In addition, even though some of our longer-term contracts contain price escalation provisions, such provisions may not fully provide for cost increases, whether from inflation, the cost of goods and services to be delivered under such contracts or otherwise.
•Research and developments—A portion of our operating expenses is associated with research and development activities associated with the design of new products. Given the specific design and application of certain of these products, there is some risk that these costs will not result in successful products in the market. Further, the timing of these products can move and be challenging to predict.
•COVID-19—COVID-19 may affect revenue growth in certain of our businesses, primarily those serving our medical end markets, and it is uncertain how materially COVID-19 will affect our global operations generally if these impacts were to persist or worsen over an extended period of time. The extent and duration of the impacts are uncertain and dependent in part on customers returning to work and economic activity ramping up. The impact of COVID-19 on our customers has affected our sales operations in certain ways, including increased customer disputes regarding orders, delayed customer notices to proceed with production, delayed payment from customers and, on rare occasions, customers have refused to pay for their orders entirely. Further, access to customer sites for sales was limited in some cases.
Non-GAAP Financial Measures
We report our financial results in accordance with generally accepted accounting principles in the United States. (“GAAP”). However, management believes certain non-GAAP financial measures provide investors and other users with additional meaningful information that should be considered when assessing our ongoing performance. Management also uses these non-GAAP financial measures in making financial, operating, and planning decisions, and in evaluating our performance. Non-GAAP financial measures should be viewed in addition to, and not as an alternative for, our GAAP results. The non-GAAP financial measures we present may differ from similarly captioned measures presented by other companies. Investors are encouraged to review the related GAAP financial measures and the reconciliation of these non-GAAP financial measures to their most directly comparable GAAP financial measures, and not to rely on any single financial measure to evaluate our business.
We use the non-GAAP financial measures “Adjusted revenues,” “Adjusted net (loss) income,” "Adjusted EPS," “EBITDA,” “EBITA,” “Adjusted EBITDA, “Free Cash Flow,” and “Adjusted Free Cash Flow.” See the “Quarterly Results of Operations” and “Cash flows” sections below for definitions of our non-GAAP financial measures and reconciliation to their most directly comparable GAAP measures.
See the "Basis of Presentation" section below regarding the Successor and Predecessor periods. The following tables present a reconciliation of certain non-GAAP financial measures for the Successor Period from October 20, 2021 through December 31, 2021 and the Predecessor Periods from July 1, 2021 through October 19, 2021, and for the fiscal years ended June 30, 2021, June 30, 2020, and June 30, 2019.
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Successor | | | Predecessor |
| From
October 20, 2021
through | | | From July 1, 2021
through | | Year Ended | | Year Ended | | Year Ended |
| December 31, 2021 | | | October 19, 2021 | | June 30, 2021 | | June 30, 2020 | | June 30, 2019 |
| ($ in millions) | Revenues | | Net Loss | | | Revenues | | Net Loss | | Revenues | | Net Loss | | Revenues | | Net Loss | | Revenues | | Net Loss |
| Total GAAP | $ | 154.1 | | | $ | (23.0) | | | | $ | 168.0 | | | $ | (105.7) | | | $ | 611.6 | | | $ | (158.4) | | | $ | 478.2 | | | $ | (119.1) | | | $ | 440.1 | | | $ | (122.0) | |
| Revenue reduction from purchase accounting | 2.3 | | | 2.3 | | | | 4.5 | | | 4.5 | | | 8.0 | | | 8.0 | | | 0.2 | | | 0.2 | | | — | | | — | |
| Cost of revenues impact from inventory valuation purchase accounting | | | 15.8 | | | | | | — | | | | | 5.2 | | | | | 1.6 | | | | | 0.1 | |
| Foreign currency (gain) loss, net | | | 1.6 | | | | | | (0.6) | | | | | 13.4 | | | | | (0.6) | | | | | (3.2) | |
| Amortization of acquired intangibles | | | 32.0 | | | | | | 19.7 | | | | | 62.8 | | | | | 50.6 | | | | | 53.0 | |
| Stock based compensation | | | 5.3 | | | | | | 9.3 | | | | | — | | | | | 0.2 | | | | | 0.1 | |
| Change in fair value of warrant liabilities | | | (1.2) | | | | | | — | | | | | — | | | | | — | | | | | — | |
| Debt extinguishment | | | — | | | | | | 15.9 | | | | | — | | | | | — | | | | | 12.8 | |
Non-operating expenses(1)(2)(3)(4)(5) | | | 7.0 | | | | | | 34.7 | | | | | 43.1 | | | | | 20.1 | | | | | 14.7 | |
| Tax impact of adjustments above | | | (14.2) | | | | | | (11.7) | | | | | (28.9) | | | | | (16.1) | | | | | (19.9) | |
| Adjusted | $ | 156.4 | | | $ | 25.6 | | | | $ | 172.5 | | | $ | (33.9) | | | $ | 619.6 | | | $ | (54.8) | | | $ | 478.4 | | | $ | (63.1) | | | $ | 440.1 | | | $ | (64.4) | |
| | | | | | | | | | | | | | | | | | | | |
| Weighted average common shares outstanding — basic and diluted | | | 180.773 | | | | | | N/A | | | | N/A | | | | N/A | | | | N/A |
| Dilutive Potential Common Shares - RSU's | | | 0.003 | | | | | | N/A | | | | N/A | | | | N/A | | | | N/A |
| Adjusted weighted average common shares — diluted | | | 180.776 | | | | | | N/A | | | | N/A | | | | N/A | | | | N/A |
| | | | | | | | | | | | | | | | | | | | |
| Adjusted EPS | | | $ | 0.14 | | | | | | N/A | | | | N/A | | | | N/A | | | | N/A |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Successor | | | Predecessor |
| ($ in millions) | From
October 20, 2021
through
December 31, 2021 | | | From July 1, 2021 through October 19, 2021 | | Year Ended June 30, 2021 | | Year Ended June 30, 2020 | | Year Ended June 30, 2019 |
| Net loss | $ | (23.0) | | | | $ | (105.7) | | | $ | (158.4) | | | $ | (119.1) | | | $ | (122.0) | |
| Interest expense, net | 6.2 | | | | 52.8 | | | 163.2 | | | 149.2 | | | 143.5 | |
| Income tax (benefit) provision | (6.8) | | | | (5.6) | | | (5.9) | | | (5.5) | | | (4.2) | |
| Amortization | 32.0 | | | | 19.7 | | | 62.8 | | | 50.6 | | | 53.0 | |
| EBITA | $ | 8.4 | | | | $ | (38.8) | | | $ | 61.7 | | | $ | 75.2 | | | $ | 70.3 | |
| Depreciation - Mirion Business Combination step-up | 1.3 | | | | — | | | — | | | — | | | — | |
| Depreciation - all other | 4.0 | | | | 6.2 | | | 20.8 | | | 17.9 | | | 16.5 | |
| EBITDA | $ | 13.7 | | | | $ | (32.6) | | | $ | 82.5 | | | $ | 93.1 | | | $ | 86.8 | |
| Stock compensation expense | 5.3 | | | | 9.3 | | | — | | | 0.2 | | | 0.1 | |
| Change in fair value of warrant liabilities | (1.2) | | | | — | | | — | | | — | | | — | |
| Debt extinguishment | — | | | | 15.9 | | | — | | | — | | | 12.8 | |
| Foreign currency (gain) loss, net | 1.6 | | | | (0.6) | | | 13.4 | | | (0.6) | | | (3.2) | |
| Revenue reduction from purchase accounting | 2.3 | | | | 4.5 | | | 8.0 | | | 0.2 | | | — | |
| Cost of revenues impact from inventory valuation purchase accounting | 15.8 | | | | — | | | 5.2 | | | 1.6 | | | 0.1 | |
Non-operating expenses(1)(2)(3)(4)(5) | 7.0 | | | | 34.7 | | | 43.1 | | | 20.1 | | | 14.7 | |
| Adjusted EBITDA | $ | 44.5 | | | | $ | 31.2 | | | $ | 152.2 | | | $ | 114.6 | | | $ | 111.3 | |
(1)Pre-tax non-operating expenses of $7.0 million for the Successor Period from October 20, 2021 through December 31, 2021 includes $1.9 million in costs to achieve integration and operational synergies, $2.2 million related to the Business Combination and costs to prepare for becoming a public company, $1.4 million of restructuring costs, and $1.0 million of costs to achieve information technology system integration and efficiency.
(2)Pre-tax non-operating expenses of $34.7 million for the Predecessor Stub Period from July 1, 2021 through October 19, 2021 includes $26.2 million related to the Business Combination and costs to prepare for becoming a public company, $4.1 million in costs to achieve integration and operational synergies, $1.5 million of restructuring costs, and $1.5 million of costs to achieve information technology system integration and efficiency.
(3)Pre-tax non-operating expenses of $43.1 million for the Predecessor Period fiscal year ended June 30, 2021 includes $14.2 million of legal and professional fees related to the Business Combination and costs to prepare for becoming a public company, $13.1 million in costs to achieve integration and operational synergies, $5.9 million of mergers and acquisition expenses, $5.5 million of restructuring costs, and $4.5 million of costs to achieve information technology system integration and efficiency.
(4)Pre-tax non-operating expenses of $20.1 million for the Predecessor Period fiscal year ended June 30, 2020 includes $10.8 million of mergers and acquisition expenses, $3.8 million of costs to achieve operational synergies, $3.4 million of costs to achieve information technology system integration and efficiency, and $1.6 million of expenses related to debt refinancing.
(5)Pre-tax non-operating expenses of $14.7 million for the Predecessor Period fiscal year ended June 30, 2019 includes $6.5 million of mergers and acquisition expenses, $2.8 million of costs to achieve information technology system integration and efficiency, $2.8 million of costs to achieve operational synergies, and $0.5 million of expenses related to debt refinancing.
The following tables present a reconciliation of non-GAAP Adjusted Revenue and Adjusted EBITDA by segment for the Successor and Predecessor Stub Period:
| | | | | | | | | | | | | | | | | | | | | | | |
| From October 20, 2021 through December 31, 2021 (Successor) |
| (in millions) | Medical | | Industrial | | Corporate & Other | | Consolidated |
| Revenues | $ | 49.2 | | | $ | 104.9 | | | $ | — | | | $ | 154.1 | |
| Revenue reduction from purchase accounting | 2.3 | | | — | | | — | | | 2.3 | |
| Adjusted Revenues | $ | 51.5 | | | $ | 104.9 | | | $ | — | | | $ | 156.4 | |
| | | | | | | |
| Income from operations | $ | (4.3) | | | $ | 1.1 | | | $ | (19.7) | | | $ | (22.9) | |
| Amortization | 13.8 | | | 18.2 | | | — | | | 32.0 | |
| Depreciation - core | 2.3 | | | 1.5 | | | 0.2 | | | 4.0 | |
| Depreciation - Mirion Business Combination step-up | 0.9 | | | 0.4 | | | — | | | 1.3 | |
| Revenue reduction from purchase accounting | 2.3 | | | — | | | — | | | 2.3 | |
| Cost of revenues impact from inventory valuation purchase accounting | 3.3 | | | 12.5 | | | — | | | 15.8 | |
| Stock based compensation | — | | | — | | | 5.3 | | | 5.3 | |
| Non-operating expenses | — | | | — | | | 6.6 | | | 6.6 | |
| Other Income / Expense | — | | | — | | | 0.1 | | | 0.1 | |
| Adjusted EBITDA | $ | 18.3 | | | $ | 33.7 | | | $ | (7.5) | | | $ | 44.5 | |
| | | | | | | | | | | | | | | | | | | | | | | |
| From July 1, 2021 through October 19, 2021 (Predecessor) |
| (in millions) | Medical | | Industrial | | Corporate & Other | | Consolidated |
| Revenues | $ | 60.3 | | | $ | 107.7 | | | $ | — | | | $ | 168.0 | |
| Revenue reduction from purchase accounting | 4.5 | | | — | | | — | | | 4.5 | |
| Adjusted Revenues | $ | 64.8 | | | $ | 107.7 | | | $ | — | | | $ | 172.5 | |
| | | | | | | |
| Income from operations | $ | 0.7 | | | $ | 11.7 | | | $ | (54.0) | | | $ | (41.6) | |
| Amortization | 9.8 | | | 9.9 | | | — | | | 19.7 | |
| Depreciation - core | 3.5 | | | 2.5 | | | 0.2 | | | 6.2 | |
| Depreciation - Mirion Business Combination step-up | — | | | — | | | — | | | — | |
| Revenue reduction from purchase accounting | 4.5 | | | — | | | — | | | 4.5 | |
| Cost of revenues impact from inventory valuation purchase accounting | — | | | — | | | — | | | — | |
| Stock based compensation | — | | | — | | | 9.3 | | | 9.3 | |
| Non-operating expenses | — | | | — | | | 33.5 | | | 33.5 | |
| Other Income / Expense | — | | | — | | | (0.4) | | | (0.4) | |
| Adjusted EBITDA | $ | 18.5 | | | $ | 24.1 | | | $ | (11.4) | | | $ | 31.2 | |
Our Business Segments
We manage and report our business in two business segments: Medical and Industrial.
Medical includes products and services for radiation therapy and personal dosimetry. This segment’s principal offerings are:
•Radiation Therapy Quality Assurance Solutions for calibrating and/or verifying imaging, treatment machine, patient treatment plan, and patient treatment accuracy (hardware and software);
•Dosimetry Solutions for monitoring the total amount of radiation medical staff members are exposed to over time; and
•Radionuclide Therapy Solutions, which includes products for nuclear medicine in radiation measurement, shielding, product handling, medical imaging furniture and rehabilitation.
Industrial includes products and services for defense, nuclear energy, laboratories and research and other industrial markets. This segment’s principal offerings are:
•Reactor Safety and Control Systems, which includes radiation monitoring systems and reactor instrumentation and control systems that ensure the safe operation of nuclear reactors and other nuclear fuel cycle facilities; and
•Radiological Search, Measurement and Analysis Systems, which includes solutions to locate, measure and perform in-depth scientific analysis of radioactive sources for radiation safety, security, and scientific applications
Recent Developments
Credit Agreement
On October 20, 2021, certain subsidiaries of the Company entered into a credit agreement (the “2021 Credit Agreement”) among Mirion Technologies (HoldingSub2), Ltd., a limited liability company incorporated in England and Wales, as Holdings, Mirion Technologies (US Holdings), Inc., as the Parent Borrower, Mirion Technologies (US), Inc., as the Subsidiary Borrower, the lending institutions party thereto, Citibank, N.A., as the Administrative Agent and Collateral Agent and Goldman Sachs Lending Partners, Citigroup Global Markets Inc., Jefferies Finance LLC and JPMorgan Chase Bank, N.A., as the Joint Lead Arrangers and Bookrunners. The 2021 Credit Agreement provides for an $830.0 million senior secured first lien term loan facility and a $90.0 million senior secured revolving facility (collectively, the “Credit Facilities”). For more information, see “—Liquidity and Capital Resources—Debt Profile.” The 2021 Credit Agreement refinanced and replaced that certain credit agreement from March 2019, by and between, among others, Mirion Technologies (HoldingRep), Ltd., its subsidiaries and Morgan Stanley Senior Funding Inc., as administrative agent, certain other revolving lenders and a syndicate of institutional lenders (the “2019 Credit Facility”). The 2021 Credit Agreement was amended by Amendment No. 1 to Credit Agreement on November 22, 2021 which permitted Holdings, Parent Borrower and its Restricted Subsidiaries (as defined in the 2021 Credit Agreement) to change their fiscal year from June 30 to December 31.
Profits Interests
In conjunction with the Business Combination Agreement, on June 17, 2021 the Sponsor issued membership interests to certain Mirion employees and the Chairman of the Board of Mirion (collectively, the "Profits Interests"). The Profits Interests are subject to service and performance vesting conditions and do not fully vest until all of the applicable conditions are satisfied. In addition, the Profits Interests are subject to certain forfeiture conditions. Accordingly, these awards have been treated as stock-based compensation under ASC Topic 718. The grant date fair value of the profits interests is based upon a valuation model using Monte Carlo simulations. As the Profits Interests included the completion of the Business Combination as a vesting condition, the expense that accumulated prior to the Business Combination was recorded on the last day of the Predecessor Stub Period and the remainder is recorded over the future vesting period.
CIRS Acquisition
On December 1, 2021, the Company purchased 100% of the issued and outstanding shares of Computerized Imaging Reference Systems, Inc. (“CIRS”) for an aggregate of $54 million, net of cash acquired of $1.0 million. CIRS is a leading provider of medical imaging and radiation therapy phantoms serving the medical industry located in the United States. The acquisition is included in our Medical segment and will advance the Company’s strategy to further expand into the medical treatment markets globally.
Other Acquisitions
During the periods presented we completed three acquisitions of U.S.-based providers of dosimetry services which we believe will increase the U.S. footprint of Mirion's industry-leading dosimetry product offering. On December 1, 2021 we acquired Safeline Monitors Systems LLC, for $1.5 million plus contingent consideration of $0.5 million. On November 1, 2021, we acquired CHP Dosimetry, for $2.5 million. On September 1, 2021 we acquired Dosimetry Badge for $1.8 million plus contingent consideration of $0.8 million.
SNC Acquisition
On December 18, 2020, we purchased 100% of the issued and outstanding shares of Sun Nuclear Corporation (“SNC”) for an aggregate of $258.1 million, net of cash acquired of $18.8 million. SNC is a global leader in radiation therapy quality assurance, delivering patient safety solutions for diagnostic imaging and radiation therapy providers in multiple countries around the world. The acquisition is included in our Medical segment and will advance the Company’s strategy to further expand into the radiation therapy markets globally.
Biodex Acquisition
On September 1, 2020, the Company purchased 100% of the issued and outstanding shares of Biodex Medical Systems, Inc. (“Biodex”) for an aggregate of $26.9 million, net of cash acquired of $4.1 million. Biodex is a manufacturer and distributor of medical devices and related replacement parts for physical and nuclear medicine, as well as medical imaging applications located in the United States. The acquisition is included in our Medical segment and will advance the Company’s strategy to further expand into the medical treatment markets globally.
Public Company Costs
We expect to continue as an SEC-registered and NYSE-listed company. We expect to hire additional staff and implement new processes and procedures to address public company requirements. We have incurred substantial additional expenses for, among other things, directors’ and officers’ liability insurance, director fees, and additional internal and external costs for investor relations, accounting, audit, legal and other functions.
Basis of Presentation
Financial information presented was derived from our historical consolidated financial statements and accounting records, and they reflect the historical financial position, results of operations and cash flows of the business in conformity with U.S. GAAP for financial statements and pursuant to the accounting and disclosure rules and regulations of the SEC. The consolidated financial statements include the accounts of the Company and its wholly owned and majority-owned or controlled subsidiaries. For consolidated subsidiaries where our ownership is less than 100%, the portion of the net income or loss allocable to noncontrolling interests is reported as “Income (Loss) attributable to noncontrolling interests” in the consolidated statements of operations. All intercompany accounts and transactions have been eliminated in consolidation. See the notes to the financial statements included in this Annual Report on Form 10-K for additional information.
As a result of the Business Combination, the Company’s financial statement presentation distinguishes Mirion TopCo as the “Predecessor” through the Closing Date. The Company, which includes the combination of GSAH and Mirion subsequent to the Business Combination, is the “Successor” for periods after the Closing Date. As a result of the application of the acquisition method of accounting in the Successor Period, the financial statements for the Successor Period are presented on a full step-up basis as a result of the Business Combination, and are therefore not comparable to the financial statements of the Predecessor Periods that are not presented on the same full step-up basis due to the Business Combination.
Certain Factors Affecting Comparability to Prior Period Financial Results
Prior to the Business Combination, GSAH operated as a special purpose acquisition company formed for the purpose of effecting a merger, capital stock exchange, asset acquisition, stock purchase, reorganization or similar business combination with one or more businesses or assets. As a result, operations were minimal before the Business Combination and are not presented in the Predecessor Periods presented prior to the Business Combination. After the Business Combination our results of operations are not directly comparable to historical results of the operations for the periods presented, primarily because:
•Mirion TopCo's fiscal year-end was changed from June 30 to December 31 to align with GSAH's fiscal year end. As a result of this change, the consolidated financial statements presented include the Predecessor Stub Period from July 1, 2021 through October 19, 2021, and the Successor Period from October 20, 2021 through December 31, 2021.
•In connection with the Business Combination, certain assets and liabilities had fair value adjustments applied to the Predecessor’s consolidated financial statements on the Closing Date, most notably:
◦Inventory;
◦Property, plant, and equipment;
◦Goodwill;
◦Intangible assets; and
◦Taxes.
•The Successor Period ended December 31, 2021 only includes Mirion's operating activity since the consummation of the Business Combination on October 20, 2021.
As a result of the factors listed above, historical results of operations and other financial data, as well as period-to-period comparisons of these results, may not be comparable or indicative of future operating results or future financial condition.
Results of Operations
The following tables set forth the Company’s results of operations for the Successor Period and the Predecessor Stub Period compared to the unaudited 4six months ended December 31, 2020 (in millions).
Periods from October 20, 2021 through December 31, 2021 (Successor) and July 1, 2021 through October 19, 2021 (Predecessor Stub Period) Compared to unaudited Six Months Ended December 31, 2020 (Predecessor)
| | | | | | | | | | | | | | | | | | | | |
| Successor | | | Predecessor | | Predecessor |
| (Dollars in millions) | From
October 20, 2021
through
December 31, 2021 | | | From July 1, 2021 through October 19, 2021 | | Six Months Ended December 31, 2020 (unaudited) |
| Revenues | $ | 154.1 | | | | $ | 168.0 | | | $ | 265.4 | |
| Cost of revenues | 100.2 | | | | 97.7 | | | 155.8 | |
| Gross profit | 53.9 | | | | 70.3 | | | 109.6 | |
| Selling, general and administrative expenses | 70.1 | | | | 101.6 | | | 84.0 | |
| Research and development | 6.7 | | | | 10.3 | | | 10.3 | |
| Income (loss) from operations | (22.9) | | | | (41.6) | | | 15.3 | |
| Interest expense, net | 6.2 | | | | 52.8 | | | 76.4 | |
| Foreign currency loss (gain), net | 1.6 | | | | (0.6) | | | 16.3 | |
| Change in fair value of warrant liabilities | (1.2) | | | | — | | | — | |
| Other expense (income), net | 0.3 | | | | 1.6 | | | (0.3) | |
| Loss on debt extinguishment | — | | | | 15.9 | | | — | |
| Loss before benefit from income taxes | (29.8) | | | | (111.3) | | | (77.1) | |
| Benefit from income taxes | (6.8) | | | | (5.6) | | | (17.4) | |
| Net loss | (23.0) | | | | (105.7) | | | (59.7) | |
| Loss attributable to noncontrolling interests | (0.8) | | | | — | | | — | |
| Net loss attributable to stockholders | $ | (22.2) | | | | $ | (105.7) | | | $ | (59.7) | |
Overview
Revenues were $154.1 million for the Successor Period from October 20, 2021 through December 31, 2021 and $168.0 million for the Predecessor Stub Period from July 1, 2021 through October 19, 2021. The increase of $56.7 million from the six months ended December 31, 2020 was primarily driven by acquisitions in the Medical segment and organic growth in the Medical segment. Cost of revenues was $100.2 million for the Successor Period and $97.7 million in the Predecessor Stub Period which increased 27.0% compared to the unaudited six months ended December 31, 2020, reflecting the increase in revenues. Gross profit increased by $14.6 million from the unaudited six months ended December 31, 2020. There was a net loss attributable to stockholders of $22.2 million for the Successor Period and $105.7 million for the Predecessor Stub Period. Net loss for the unaudited six months ended December 31, 2020 was $59.7 million. There was a $68.2 million increase in net loss as a result of the increase in gross profit, more than offset by higher SG&A expenses of $87.7 million, primarily driven by the impact of acquisitions in the Medical segment, increased non-operational legal and professional fees incurred to prepare for being a public company, and stock-based compensation expense. Offsetting the increase in net loss period over period was decreased net interest expense of $17.4 million, the positive impact of foreign currency exchange of $15.3 million, a gain recognized on the change in fair value of warrant liabilities of $1.2 million, offset by a net increase in income tax benefit of $5.0 million and a net increase in other expense (income), net of $2.3 million. The impact of purchase accounting related to the fair value adjustment of deferred revenue for the SNC acquisition reduced revenue for the Successor and Predecessor Stub Periods by $6.8 million. The impact of purchase accounting related to the fair value of inventory increased cost of revenues by $15.8 million for the Successor Period and $0.5 million for the unaudited six months ended December 31, 2020. The impact of purchase accounting related to the fair values of intangible assets and property, plant, and equipment for the Business Combination resulted in increased amortization and depreciation expense and increased net loss by $18.7 million and $1.3 million, respectively.
Revenues
Revenues were $154.1 million for the Successor Period and $168.0 million for the Predecessor Stub Period. Revenues increased $56.7 million from the unaudited six months ended December 31, 2020. The majority of the increase was a result of the acquisitions in the Medical segment contributing $61.3 million (of which $53.9 million was generated by SNC, $5.1 million by Biodex, $1.5 million by CIRS, and $0.8 million by other acquisitions) and a $3.2 million increase due to organic growth. Industrial segment revenues decreased $0.7 million, primarily driven by foreign exchange rate fluctuations of $2.5 million offset by a $1.8 million increase due to organic growth. The impact of purchase accounting related to the fair value adjustment of deferred revenue for the SNC acquisition reduced Medical segment revenues for the Successor and Predecessor Stub Periods by $2.3 million and $4.5 million, respectively.
By segment, revenues for the Successor and Predecessor Period were $49.2 million and $60.3 million in the Medical segment, respectively, and $104.9 million and $107.7 million in the Industrial segment for the Successor and Predecessor Periods, respectively. Movements in revenues by segment are detailed in the “Business Segments” section below.
Cost of revenues
Cost of revenues was $100.2 million for the Successor Period and $97.7 million for the Predecessor Stub Period. Cost of Revenues for the unaudited six months ended December 31, 2020 were $155.8 million. Cost of revenues as a percentage of revenues was 65.0% for the Successor Period, 58.2% for the Predecessor Stub Period, and 58.7% for the six months ended December 31, 2020. The increase in the Successor Period was driven by purchase accounting related to the fair value of inventory from the Business Combination. In addition, cost of revenues increased over the unaudited six months ended December 31, 2020 due to acquisitions in our Medical segment ($25.9 million combined from SNC, Biodex, CIRS, and other acquisitions) and an increase in our Industrial segment cost of revenues of $0.7 million offset by a decrease due to the impacts from foreign currency exchange rate fluctuations of $1.9 million. Cost of revenues for the Successor Period includes $15.8 million due to purchase accounting related to the fair value of inventory from the Business Combination, $0.9 million of increased amortization expense resulting from increased intangible assets from the Business Combination, and $1.1 million of increased depreciation expense resulting from increased fair values of property, plant, and equipment from the Business Combination. Cost of revenues for the unaudited six months ended December 31, 2020 includes $0.5 million due to purchase accounting related to the fair value of inventory from previous acquisitions.
Selling, general and administrative expenses
Selling, general and administrative (“SG&A”) expenses were $70.1 million for the Successor Period and $101.6 million for the Predecessor Stub Period. SG&A expenses were $84.0 million for the unaudited six months ended December 31, 2020, resulting in an increase of $87.7 million. The primary drivers behind the increase in SG&A expenses were the impact of acquisitions in the Medical segment ($20.8 million combined from SNC, Biodex, and CIRS), a $28.4 million increase related to the Business Combination and costs to prepare for becoming a public company, and a $14.6 million increase in stock based compensation expense related to the Profits Interests (see Note 14, Stock-based compensation). SG&A for the Successor Period includes $17.8 million of increased amortization expense resulting from increased intangible assets from the Business Combination and $0.2 million of increased depreciation expense resulting from increased fair values of property, plant, and equipment from the Business Combination.
Research and development
Research and development (“R&D”) expenses were $6.7 million for the Successor Period and $10.3 million for the Predecessor Stub Period. R&D expenses were $10.3 million for the unaudited six months ended December 3, 2020, resulting in an increase of $6.7 million. The increase in R&D expense was primarily a result of the prior period acquisitions in our Medical segment ($9.7 million combined from SNC, Biodex, and CIRS), partially offset by a decrease in R&D activity expensed in our Industrial segment of $2.2 million.
Income (loss) from operations
Loss from operations was $22.9 million for the Successor Period and $41.6 million for the Predecessor Stub Period. Income from operation was $15.3 million during the unaudited six months ended December 31, 2020 which resulted in an increased loss of $79.8 million. On a segment basis, income (loss) from operations in the Medical segment for the Successor Period and Predecessor Stub Period was $(4.3) million and $0.7 million, respectively, which includes $16.2 million and $4.5 million, respectively, in purchase accounting impacts described in revenues, cost of revenues, and SG&A above. Income from operations in the Industrial segment for the Successor Period and Predecessor Stub Period was $1.1 million and $11.7 million, respectively, which includes $21.4 million in purchase accounting impacts described in cost of
revenues and SG&A above in the Successor Period. Corporate expenses were $19.7 million and $54.0 million for the Successor Period and Predecessor Stub Period, respectively. See “Business segments” and “Corporate and other” below for further details.
Interest expense, net
Interest expense, net, was $6.2 million for the Successor Period and $52.8 million for the Predecessor Stub Period. Interest expense, net, was $76.4 million for the unaudited six months ended December 31, 2020. The $17.4 million change is a non-cash decrease in interest related to the Shareholder Notes which were paid in full in connection with the closing of the Business Combination. See Note 8, Borrowings, to the consolidated financial statements included elsewhere in this Annual Report on Form 10-K.
Loss on debt extinguishment
Loss on debt extinguishment in the Predecessor Stub Period of $15.9 million is the result of the write-off of previously deferred financing costs related to the 2019 Credit Facility that was extinguished in connection with the Business Combination. See Note 8, Borrowings, to the consolidated financial statements included elsewhere in this Annual Report on Form 10-K.
Foreign currency (gain) loss, net
The Company recorded a loss of $1.6 million for the Successor Period and a gain of $0.6 million for the Predecessor Stub Period from foreign currency exchange. The Company recorded a loss of $16.3 million for the unaudited six months ended December 31, 2020, from foreign currency exchange. The change in net foreign currency losses is due to appreciation in European and Canadian local currencies in relation to the U.S. dollar and primarily related to our Euro debt in the prior year comparable period.
Change in fair value of warrant liabilities
The Company recognized an unrealized gain of $1.2 million resulting from an increase in the fair value of the Public Warrant and Private Placement Warrant liabilities during the Successor Period. See Note 17, Fair Value Measurements, to the consolidated financial statements included elsewhere in this Annual Report on Form 10-K.
Income taxes
Income tax benefit was $6.8 million for the Successor Period and $5.6 million for the Predecessor Stub Period. Income tax benefit was $17.4 million for the unaudited six months ended December 31, 2020. Income tax benefit in the Successor Period differed from income tax benefit in the Predecessor Stub Period and the unaudited six months ended December 31, 2020, primarily due to changes in valuation allowances.
Business segments
The following provides detail for business segment results for the Successor Period, the Predecessor Stub Period, and the unaudited six months ended December 31, 2020. Segment income from operations includes revenues of the segment less expenses that are directly related to those revenues but excludes certain charges to cost of revenues and SG&A expenses predominantly related to corporate costs, shared overhead and other costs related to restructuring activities and costs to achieve operational initiatives, which are included in Corporate and Other in the table below. Interest expense, loss on debt extinguishment, foreign currency loss (gain), net, and other expense (income), net, are not allocated to segments.
For reconciliations of segment revenues and operating income to our consolidated results, see Note 16, Segment Information, to the consolidated financial statements included elsewhere in this Annual Report on Form 10-K.
Medical
| | | | | | | | | | | | | | | | | | | | | | | |
| Successor | | | Predecessor | | | |
| (Dollars in millions) | From
October 20, 2021
through
December 31, 2021 | | | From July 1, 2021 through October 19, 2021 | | | Six Months Ended December 31, 2020 (unaudited) |
| Revenues | $ | 49.2 | | | | $ | 60.3 | | | | $ | 52.1 | |
| Income (loss) from operations | $ | (4.3) | | | | $ | 0.7 | | | | $ | 8.7 | |
| Income (loss) from operations as a % of revenues | (8.7) | % | | | 1.2 | % | | | 16.7 | % |
Medical segment revenues were $49.2 million for the Successor Period and $60.3 million for the Predecessor Stub Period, which is an increase of $57.4 million from Medical segment revenues of $52.1 million for the unaudited six months ended December 31, 2020. Revenues increased primarily due to the impact of acquisitions contributing $61.3 million (of which $53.9 million was generated by SNC, $5.1 million by Biodex, $1.5 million by CIRS, and $0.8 million by other acquisitions) and an increase of $3.2 million in our legacy business due to organic growth. Additionally, foreign currency exchange rates positively impacted Medical revenues by approximately $0.3 million. The impact of purchase accounting related to the fair value adjustment of deferred revenue for the SNC acquisition reduced Medical segment revenues for the Successor and Predecessor Stub Periods by $2.3 million and $4.5 million, respectively.
Loss from operations, which excludes non-operational costs, was $4.3 million for the Successor Period and income from operations was $0.7 million for the Predecessor Stub Period. Income from operations, which excludes non-operational costs, was $8.7 million for the unaudited six months ended December 31, 2020, representing a decrease in income from operations of $12.3 million. Income from operations as a percentage of revenues decreased approximately 20.0% largely due to the lower margins and higher operating expenses of acquisitions, driven in large part by amortization expense (reducing margins by $1.6 million and increasing operating expenses by $5.8 million). Additionally, income from operations as a percentage of revenues was impacted in the Successor Period by a $2.3 million reduction in revenue resulting from purchase accounting related to the SNC acquisition; increases in cost of revenues resulting from the purchase accounting impacts on inventory, amortization, and depreciation in connection with the Business Combination of $3.2 million, $2.0 million, and $0.8 million, respectively; and increases in SG&A expenses resulting from the purchase accounting impacts on amortization and depreciation in connection with the Business Combination of $8.3 million and $0.1 million, respectively. Income from operations as a percentage of revenues was impacted in the Predecessor Stub Period by a $4.5 million reduction in revenue resulting from purchase accounting related to the SNC acquisition, and in the unaudited six months ended December 31, 2020 by a $0.5 million due to purchase accounting related to the fair value of inventory from previous acquisitions.
Industrial
| | | | | | | | | | | | | | | | | | | | | | | |
| Successor | | | Predecessor | | | |
| (Dollars in millions) | From
October 20, 2021
through
December 31, 2021 | | | From July 1, 2021 through October 19, 2021 | | | Six Months Ended December 31, 2020 (unaudited) |
| Revenues | $ | 104.9 | | | | $ | 107.7 | | | | $ | 213.3 | |
| Income from operations | $ | 1.1 | | | | $ | 11.7 | | | | $ | 33.8 | |
| Income from operations as a % of revenues | 1.0 | % | | | 10.9 | % | | | 15.8 | % |
Industrial segment revenues were $104.9 million for the Successor Period and $107.7 million for the Predecessor Stub Period, which was a slight decrease of $0.7 million from revenues of $213.3 million for the unaudited six months ended December 31, 2020. The slight decrease is primarily driven by foreign exchange rate fluctuations of $2.5 million offset by a $1.8 million increase due to organic growth.
Income from operations, which excludes non-operational costs, was $1.1 million for the Successor Period and $11.7 million for the Predecessor Stub Period. Income from operations, which excludes non-operational costs, was $33.8 million for the period ending December 31, 2020, representing a decrease of $21.0 million driven primarily by higher cost of revenues including $12.6 million of inventory step-up and higher amortization of $8.4 million, both related to the Business Combination purchase accounting.
Corporate and other
Corporate and other costs include costs associated with our corporate headquarters located in Georgia, as well as centralized global functions including Executive, Finance, Legal and Compliance, Human Resources, Technology, Strategy, and Marketing and other costs related to company-wide initiatives (e.g., Business Combination transaction expenses, merger and acquisition activities, restructuring and other initiatives).
Corporate and other costs were $19.7 million for the Successor period and $54.0 million for the Predecessor Stub Period which is an increase of $46.7 million when compared to the unaudited six months ended December 31, 2020. The increase versus the comparable period was predominantly driven by $28.4 million of fees related to the Business Combination and costs to prepare for becoming a public company, an increase in stock-based compensation expense of $14.6 million related to the Profits Interests (see Note 14, Stock based compensation), $2.0 million increase in other costs related to company-wide initiatives ($4.2 million in operations and information technology integrations, $1.6 million in restructuring costs, partially offset by $3.6 million in lower merger and acquisition costs), an increase of $1.8 million in compensation expense, $0.9 million increase in facility costs and $0.9 million increase in corporate insurance mostly due to becoming a public company. For reconciliations of segment operating income and corporate and other costs to our consolidated results, see Note 16, Segment Information, to the consolidated financial statements included elsewhere in this Annual Report on Form 10-K.
Year ended June 30, 2021 (Predecessor) compared to year ended June 30, 2020 (Predecessor)
| | | | | | | | | | | | | | | | | | | | | | | |
| (Dollars in millions) | 2021 | | 2020 | | $ Change | | % Change |
| Revenues | $ | 611.6 | | | $ | 478.2 | | | $ | 133.4 | | | 27.9 | % |
| Cost of revenues | 359.8 | | | 281.2 | | | 78.6 | | | 28.0 | % |
| Gross profit | 251.8 | | | 197.0 | | | 54.8 | | | 27.8 | % |
| Selling, general and administrative expenses | 211.2 | | | 158.1 | | | 53.1 | | | 33.6 | % |
| Research and development | 29.4 | | | 15.9 | | | 13.5 | | | 84.9 | % |
| Income from operations | 11.2 | | | 23.0 | | | (11.8) | | | (51.3) | % |
| Interest expense, net | 163.2 | | | 149.2 | | | 14.0 | | | 9.4 | % |
| Foreign currency loss (gain), net | 13.4 | | | (0.6) | | | 14.0 | | | N/A |
| Other (income) expense, net | (1.1) | | | (1.0) | | | (0.1) | | | 10.0 | % |
| Loss before benefit from income taxes | (164.3) | | | (124.6) | | | (39.7) | | | 31.9 | % |
| Benefit from income taxes | (5.9) | | | (5.5) | | | (0.4) | | | 7.3 | % |
| Net loss | (158.4) | | | (119.1) | | | (39.3) | | | 33.0 | % |
| Income (loss) attributable to noncontrolling interests | (0.1) | | | — | | | (0.1) | | | N/A |
| Net loss attributable to stockholders | (158.3) | | | (119.1) | | | $ | (39.2) | | | 32.9 | % |
Overview
Revenues for the year ended June 30, 2021 (“FY 2021” or “fiscal 2021”) were $611.6 million, resulting in an increase of $133.4 million, or 27.9%, from the same period in the prior year primarily driven by acquisitions in the Medical segment and organic growth in the Industrial segment. Cost of revenues of $359.8 million also increased 28.0% compared to the same period in the prior year reflecting the increase in revenues, a $3.1 million increase in restructuring costs, and a $3.4 million increase in costs to achieve operational synergies. Gross profit increased by $54.8 million and as a percentage of revenue was consistent period over period for the Company, including a decrease in percentage of revenue in our Medical segment of 8.5%, offset by an increase in percentage of revenue in our Industrial segment of 1.4%. There was a net loss of $158.4 million for the year ended June 30, 2021 compared to a net loss of $119.1 million during the year ended June 30, 2020. The $39.3 million, or 33.0%, increase is the result of the increase in gross profit, offset by higher SG&A expenses of $53.1 million, primarily driven by acquisitions in the Medical segment and a $17.6 million increase in non-operational legal and professional fees incurred to prepare for being a public company and costs related to restructuring, mergers and acquisitions and costs to achieve synergies. Also contributing to the increase in net loss period over period was increased net interest expense of $14.0 million and the negative impact of foreign currency exchange of $14.0 million offset by a net increase in income tax benefit of $0.4 million. The impact of purchase accounting related to the fair value adjustment of
deferred revenue reduced revenue in the year ended June 30, 2021 by $8.0 million. The impact of purchase accounting related to the fair value of inventory increased cost of revenues by $5.2 million for the year ended June 30, 2021.
Revenues
Revenues were $611.6 million for the year ended June 30, 2021, an increase of $133.4 million, or 27.9%, compared with $478.2 million for the year ended June 30, 2020. The majority of the increase was a result of the acquisitions in the Medical segment contributing $91.7 million (of which $48.9 million was generated by SNC, $32.6 million by Biodex, $9.2 million from AWST and $1.0 million from Dosimetrics). The Industrial segment revenues also increased $39.7 million of which $11.4 million was driven by Reactor Safety and Control Systems products and $28.3 million was driven by Radiological Search, Measurement, and Analysis Systems products resulting from increased product orders and release of new products and the positive impact from foreign currency exchange rate fluctuations of $18.4 million. The impact of purchase accounting related to the fair value adjustment of deferred revenue reduced revenue in the year ended June 30, 2021 by $8.0 million.
By segment, revenues for the year ended June 30, 2021 were $155.7 million in the Medical segment and $455.9 million in the Industrial segment. Movements in revenues by segment are detailed in the Business Segments section below.
Cost of revenues
Cost of revenues was $359.8 million for the year ended June 30, 2021, an increase of $78.6 million, or 28.0% compared to the year ended June 30, 2020. Cost of revenues as a percentage of revenues was flat year over year. The increase in cost of revenues was primarily due to acquisitions in our Medical segment ($53.6 million combined from SNC, Biodex, AWST, and Dosimetrics), an increase in our Industrial segment cost of revenues of $17.5 million related to the increase in revenues, including the impacts from foreign currency exchange rate fluctuations of $10.9 million, and $6.5 million of restructuring costs and costs to achieve operational synergies. Cost of revenues includes a $5.2 million increase from purchase accounting related to the fair value of inventory for the year ended June 30, 2021.
Selling, general and administrative expenses
Selling, general and administrative (“SG&A”) expenses were $211.2 million for the year ended June 30, 2021, an increase of $53.1 million, or 33.6%, compared to the year ended June 30, 2020. SG&A expenses as a percentage of revenues were 34.5% for the twelve months ended June 30, 2021, a 1.5 percentage point increase compared with 33.1% for the twelve months ended June 30, 2020. The primary drivers behind the increase in SG&A expenses were the impact of acquisitions in the Medical Segment ($34.5 million combined from SNC, Biodex, AWST and Dosimetrics), $17.6 million increase in non-operational legal and professional fees incurred to prepare for being a public company and costs related to restructuring, mergers and acquisitions and costs to achieve synergies, $6.4 million increase in compensation-related expenses and the impact from foreign currency exchange rate fluctuations of $5.5 million, partially offset by a decrease in amortization of $3.7 million and a decrease in travel and entertainment expenses of $3.7 million.
Research and development
Research and development (“R&D”) expenses were $29.4 million for the year ended June 30, 2021, an increase of $13.5 million, or 84.9%, compared to the year ended June 30, 2020. The increase in R&D expense was primarily due to business combinations ($10.4 million combined from SNC, Biodex, AWST, and Dosimetrics), increased R&D activity of $2.5 million to develop new products in the Industrial segment and the impact from foreign currency exchange rate fluctuations of $0.6 million.
Income from operations
Income from operations for the year ended June 30, 2021 was $11.2 million, a decrease of $11.8 million, or 51.3%, when compared to income from operations of $23.0 million for the year ended June 30, 2020. On a segment basis, income from operations was $6.0 million in the Medical segment, which includes $13.2 million in purchase accounting impacts described in revenues and cost of revenues above, and $81.5 million in Industrial segment. Corporate expenses were $76.3 million for the year ended June 30, 2021. See “Business segments” and “Corporate and other” below for further details.
Interest expense
Interest expense, net, was $163.2 million for the year ended June 30, 2021 compared to $149.2 million for the year ended June 30, 2020. The $14.0 million, or 9.4%, change is a non-cash increase in interest related to the Stockholder Notes which are described in Note 8 to the consolidated financial statements.
Foreign currency (gain) loss, net
The Company recorded a loss of $13.4 million for the year ended June 30, 2021, compared to a gain of $0.6 million for the year ended June 30, 2020, from foreign currency exchange. The change in net foreign currency losses is due to appreciation in European and Canadian local currencies in relation to the U.S. dollar.
Income taxes
Income tax benefit was $5.9 million for the year ended June 30, 2021 versus a benefit of $5.5 million in for the year ended June 30, 2020. The $0.4 million change is primarily due to the mix of earnings and jurisdictions during each respective period.
Business segments
The following provides detail for business segment results for the years ended June 30, 2021 and June 30, 2020. Segment income from operations includes revenues of the segment less expenses that are directly related to those revenues but excludes certain charges to cost of revenues and selling, general and administrative expenses predominantly related to corporate costs, shared overhead and other costs related to restructuring activities and costs to achieve operational initiatives, which are included in Corporate and Other in the table below. Interest expense, loss on debt extinguishment, foreign currency loss (gain), net, and other expense (income), net, are not allocated to segments. For reconciliations of segment revenues and operating income to our consolidated results, see Note 16, Segment Information, to the consolidated financial statements included elsewhere in this Annual Report on Form 10-K.
Medical
| | | | | | | | | | | | | | | | | | | | | | | |
| (Dollars in millions) | June 30,
2021 | | June 30,
2020 | | $ Change | | % Change |
| Revenues | $ | 155.7 | | | $ | 62.6 | | | $ | 93.1 | | | 148.7 | % |
| Income from operations | $ | 6.0 | | | $ | 13.9 | | | $ | (7.9) | | | (56.8) | % |
| Income from operations as a % of revenues | 3.9 | % | | 22.2 | % | | | | |
Medical segment revenues were $155.7 million, for the year ended June 30, 2021, which is an increase of $93.1 million, or 148.7%, from the year ended June 30, 2020. Revenues increased primarily due to the impact of acquisitions contributing $91.7 million (of which $48.9 million was generated by SNC, $32.6 million by Biodex, $9.2 million from AWST and $1.0 million from Dosimetrics) and an increase of $1.2 million in our legacy business. Additionally, foreign currency exchange rates positively impacted Medical revenues by approximately $0.2 million. The impact of purchase accounting related to the fair value adjustment of deferred revenue reduced revenue for the year ended June 30, 2021 by $8.0 million.
Income from operations, which excludes non-operational costs, for the year ended June 30, 2021 was $6.0 million, a decrease of $7.9 million compared with the year ended June 30, 2020. Income from operations as a percentage of revenues decreased approximately 18.3% primarily due to the lower margins and higher operating expenses of the acquisitions in the year ended June 30, 2021, driven in large part by amortization expense (reducing margins by $3.3 million and increasing operating expenses by $12.3 million). Bad debt expense in our legacy business also increased (partially driven by COVID 19) by $1.3 million. Additionally, income from operations as a percentage of revenues was impacted by the $8.0 million reduction in revenue and $5.2 million increase in cost of revenues resulting from purchase accounting.
Industrial
| | | | | | | | | | | | | | | | | | | | | | | |
| Dollars in millions) | June 30,
2021 | | June 30,
2020 | | $ Change | | % Change |
| Revenues | $ | 455.9 | | | $ | 415.6 | | | $ | 40.3 | | | 9.7 | % |
| Income from operations | $ | 81.5 | | | $ | 59.6 | | | $ | 21.9 | | | 36.7 | % |
| Income from operations as a % of revenues | 17.9 | % | | 14.3 | % | | | | |
Industrial segment revenues were $455.9 million for the year ended June 30, 2021, an increase of $40.3 million, or 9.7% from the year ended June 30, 2020. Revenues increased in both product and service revenues, primarily due to new product offerings in the Radiological Search, Measurement and Analysis Systems product group such as the MBD-2 dosimeter and Aegis spectrometer. Foreign currency positively impacted revenues by approximately $18.4 million. Additionally, revenues increased due to the impact of the acquisition of Selmic in fiscal 2020, which contributed approximately $3.6 million of additional revenue in fiscal 2021 compared with fiscal 2020.
Income from operations, which excludes non-operational costs, was $81.5 million for the year ended June 30, 2021, an increase of $21.9 million compared with the year ended June 30, 2020 driven primarily by higher revenues. Income from operations as a percentage of revenues increased 3.6% primarily due to operating expense savings driven primarily by COVID-19 restrictions on employee travel and fixed overhead absorption.
Corporate and other
Corporate and other costs include costs associated with our headquarters located in Georgia, as well as centralized global functions including Executive, Finance, Legal and Compliance, Human Resources, Technology, Strategy, and Marketing and other costs related to company-wide initiatives (e.g., merger and acquisition activities, restructuring and other initiatives). Corporate and other costs were $76.3 million and $50.5 million for the years ended June 30, 2021 and June 30, 2020, respectively. The $25.8 million increase in corporate and other expenses during the year ended June 30, 2021 versus the comparable period was predominantly driven by $14.2 million of legal and professional fees related to the Business Combination and costs to prepare for becoming a public company, an increase in compensation and related costs of $4.2 million, restructuring costs of $5.5 million, an increase in mergers and acquisition, integration and operational efficiency costs of $4.0 million, an increase in professional fees of $2.4 million, and an increase in costs to achieve information technology system integration and efficiency of $1.1 million, partially offset by a decrease in debt issuance costs of $1.6 million, a decrease in travel and entertainment expenses of $1.1 million, and a decrease in facilities costs of $1.0 million. For reconciliations of segment operating income and corporate and other costs to our consolidated results, see Note 16–Segment Information to the consolidated financial statements included elsewhere in this Annual Report on Form 10-K.
Year ended June 30, 2020 (Predecessor) compared to year ended June 30, 2019 (Predecessor)
| | | | | | | | | | | | | | | | | | | | | | | |
| (Dollars in millions) | 2020 | | 2019 | | $ Change | | % Change |
| Revenues | $ | 478.2 | | | $ | 440.1 | | | $ | 38.1 | | | 8.7 | % |
| Cost of revenues | 281.2 | | | 251.9 | | | 29.3 | | | 11.6 | % |
| Gross profit | 197.0 | | | 188.2 | | | 8.8 | | | 4.7 | % |
| Selling, general and administrative expenses | 158.1 | | | 145.4 | | | 12.7 | | | 8.7 | % |
| Research and development | 15.9 | | | 14.0 | | | 1.9 | | | 13.6 | % |
| Income from operations | 23.0 | | | 28.8 | | | (5.8) | | | (20.1) | % |
| Interest expense, net | 149.2 | | | 143.5 | | | 5.7 | | | 4.0 | % |
| Loss on extinguishment of debt | — | | | 12.8 | | | (12.8) | | | (100.0) | % |
| Foreign currency gain, net | (0.6) | | | (3.2) | | | 2.6 | | | (81.3) | % |
| Other (income) expense, net | (1.0) | | | 1.9 | | | (2.9) | | | (152.6) | % |
| Loss before benefit from income taxes | (124.6) | | | (126.2) | | | 1.6 | | | (1.3) | % |
| Benefit from income taxes | (5.5) | | | (4.2) | | | (1.3) | | | 31.0 | % |
| Net loss | (119.1) | | | (122.0) | | | 2.9 | | | (2.4) | % |
| Income (loss) attributable to noncontrolling interests | — | | | — | | | — | | | N/A |
| Net loss attributable to stockholders | (119.1) | | | (122.0) | | | $ | 2.9 | | | (2.4) | % |
Overview
Revenues for the year ended June 30, 2020 (“FY 2020” or “fiscal 2020”) were $478.2 million, an increase of 8.7% from the year ended June 30, 2019 (“FY 2019” or “fiscal 2019”) primarily driven by acquisitions in FY 2020 in both the Medical and Industrial segment. Cost of revenues increased $29.3 million, or 11.6% primarily reflecting the increase in revenues. Gross profit increased by $8.8 million and as a percentage of revenue for the Company was consistent period over period. There was a net loss of $119.1 million in FY 2020 compared to net loss of $122.0 million in FY 2019. The 2.4% decrease in net loss in FY 2020 is primarily the result of an increase in gross profit, offset by an increase in SG&A of $12.7 million, including $7.6 million of acquisition costs and costs to achieve operational synergies, decreased loss on debt extinguishment of $12.8 million and increase in other income of $2.9 million, increase in interest expense of $5.7 million, and the negative impact of foreign currency exchange of $2.6 million. The impact of purchase accounting related to the fair value of inventory increased our cost of revenues by $1.6 million for FY 2020.
Revenues
Revenues were $478.2 million for FY 2020, an increase of $38.1 million, or 8.7%, compared with $440.1 million for FY 2019. The increase in revenues was primarily due to the impact of FY 2020 acquisitions in both the Medical and Industrial segments ($31.7 million combined from Capintec, Selmic, Premium Analyse, and AWST) and higher volumes in legacy operations.
By segment, revenues were $62.6 million in the Medical segment and $415.6 million in the Industrial segment. Movements in revenues by segment are discussed in greater detail in the “Business segment” discussion below.
Cost of revenues
Cost of revenues was $281.2 million in FY 2020, an increase of $29.3 million, or 11.6% compared to FY 2019. Cost of revenues as a percentage of revenues was 58.8% for FY 2020, a 1.6% increase compared with 57.2% for FY 2019. The increase in cost of revenues was primarily due to the impact of business combinations ($22.9 million combined from Capintec, Selmic, Premium Analyse, and AWST) and unfavorable product sales mix (i.e., higher sales of products with lower margin versus products with higher margin during the period). The impact of purchase accounting related to the fair value of inventory increased our cost of revenues by $1.6 million in FY 2020.
Selling, general and administrative expenses
SG&A expenses were $158.1 million in FY 2020, an increase of $12.7 million, or 8.7%, compared to FY 2019. SG&A expenses as a percent of revenues was 33.1% in FY 2020, compared to 33.0% in FY 2019. The primary drivers behind the
increase in SG&A expenses were expenses associated with business combinations ($7.1 million combined from Capintec, Selmic, Premium Analyse, and AWST), an increase of $7.6 million in costs to achieve operational synergies and mergers and acquisitions, increase in professional fees of $2.1 million, partially offset by a decrease in amortization expense of $4.3 million.
Research and development
R&D expenses were $15.9 million in FY 2020, an increase of $1.9 million, or 13.6%, compared to FY 2019. The increase in R&D expenses was primarily due to business combinations ($1.3 million combined from Capintec, Selmic, Premium Analyse, and AWST) and increased R&D activity in existing businesses to develop new products of $0.9 million.
Income (loss) from operations
Income from operations in FY 2020 was $23.0 million, a decrease of $5.8 million, or 20.1%, when compared to income from operations of $28.8 million in FY 2019. On a segment basis, income from operations was $13.9 million in the Medical segment and $59.6 million in the Industrial segment in FY 2020 compared to $10.2 million in Medical and $55.0 million in Industrial in FY 2019. Corporate expenses were $50.5 million in FY 2020 compared to $36.4 million in FY 2019. See “Business segments” and “Corporate and other” below for further details.
Interest expense
Interest expense, net was $149.2 million in FY 2020 and $143.5 million in FY 2019. The $5.7 million, or 4.0%, increase in interest expense in FY 2020 was due primarily to increased non-cash interest of $11.9 million on related-party Shareholder Notes, partially offset by a decrease in interest expense related to our third-party debt due to lower interest rates on third-party debt. See Note 8–Borrowings to consolidated financial statements included elsewhere in this Annual Report on Form 10-K.
Loss on debt extinguishment
There was no loss on debt extinguishment in FY 2020, as compared to the FY 2019 loss on extinguishment of $12.8 million. In FY 2019, we entered into a new credit agreement, resulting in the extinguishment of previous debt. No similar debt extinguishment occurred in FY 2020.
Foreign currency (gain) loss, net
The Company recorded a gain of $0.6 million in FY 2020, compared to a gain of $3.2 million from foreign currency exchange in FY 2019. Foreign currency gain decreased $2.6 million, or 81.3%, primarily due to less favorable exchange rates in FY 2020 between the U.S. dollar and currencies used in our European operations.
Other (income) expenses, net
Other income was $1.0 million in FY 2020, compared to other expense of $1.9 million in FY 2019. The change in other (income) expenses, net from FY 2019 is primarily due to investment income received in FY 2020 compared to losses recorded on the disposal of property, plant, and equipment in FY 2019.
Income taxes
Income tax benefit was $5.5 million in FY 2020 as compared to income tax benefit of $4.2 million in FY 2019, which increased $1.3 million due to the impact of the release of unrecognized tax benefits related to uncertain tax positions offset by increases in valuation allowances and mix of income between U.S. and foreign operations.
Business segments
The following is an analysis of business segment results for FY 2020 as compared with FY 2019. Segment income from operations is defined as revenues less cost of revenues, segment selling, general and administrative expenses, and research and development expenses. Costs not specifically allocated to segment operating include those discussed in further detail in the Corporate and other section below. Interest expense, loss on debt extinguishment, foreign currency gain, and other income/expense are not allocated to segments. For reconciliations of segment revenues and earnings to our consolidated results, see Note 16–Segment Information to the consolidated financial statements included elsewhere in this Annual Report Form 10-K.
Medical
| | | | | | | | | | | | | | | | | | | | | | | |
| (Dollars in millions) | June 30,
2020 | | June 30,
2019 | | $ Change | | % Change |
| Revenues | $ | 62.6 | | | $ | 42.9 | | | $ | 19.7 | | | 45.9 | % |
| Income from operations | $ | 13.9 | | | $ | 10.2 | | | $ | 3.7 | | | 36.3 | % |
| Income from operations as a % of revenues | 22.2 | % | | 23.8 | % | | | | |
Medical revenues were $62.6 million in FY 2020, an increase of $19.7 million or 45.9% from FY 2019 primarily due to the impact of business combinations ($17.7 million from Capintec and AWST in FY 2020 and $0.9 million from the full fiscal year impact of the NRG Dosimetry Services Group (“NRG”) FY 2019 acquisition).
Income from operations was $13.9 million in FY 2020, representing an increase in earnings of $3.7 million, or 36.3%, from FY 2019 primarily due to the impact of business combinations ($1.3 million from Capintec and AWST), higher gross profit from legacy operations of $1.5 million due to product mix, and lower amortization expense related to legacy operations of $0.7 million. Income from operations as a percentage of revenues declined 1.6% primarily due to the product mix impact of business combinations, as certain products had lower margins than our legacy medical businesses.
Industrial
| | | | | | | | | | | | | | | | | | | | | | | |
| (Dollars in millions) | June 30,
2020 | | June 30,
2019 | | $ Change | | % Change |
| Revenues | $ | 415.6 | | | $ | 397.2 | | | $ | 18.4 | | | 4.6 | % |
| Income from operations | $ | 59.6 | | | $ | 55.0 | | | $ | 4.6 | | | 8.4 | % |
| Income from operations as a % of revenues | 14.3 | % | | 13.8 | % | | | | |
Industrial revenues were $415.6 million in FY 2020, an increase of $18.4 million, or 4.6% from FY 2019. Revenues increased primarily due to business combinations ($14.0 million from Selmic and Premium Analyse), new product offerings, and government year-end purchases driving increased revenues from certain customers.
Income from operations, which includes an inventory valuation impact of $1.3 million but excludes non-operational costs, was $59.6 million in FY 2020, an increase of $4.6 million, or 8.4%, compared with the prior year period, while income from operations as a percentage of revenues increased 0.5%. The $4.6 million increase in income from operations was primarily due to the impact of business combinations ($1.3 million from Selmic and Premium Analyse), lower amortization expense related to legacy operations of $3.4 million and reduced travel expenses of $1.3 million, offset by lower gross profit impact of approximately $1.4 million from legacy operations due to product mix.
Corporate and other
Corporate and other costs include costs associated with our headquarters, as well as centralized global functions including Executive, Finance, Legal and Compliance, Human Resources, Technology, Strategy, and Marketing. Corporate and other costs were $50.5 million and $36.4 million in the 2020 and 2019 periods, respectively. The $14.1 million increase in corporate and other expenses in FY 2020 versus the comparable prior year period was primarily the result of $8.8 million increase in costs to achieve synergies, acquisition, integration and strategic initiatives, an increase of $2.0 million in compensation and related costs and $1.8 million increase in professional fees. For reconciliations of segment operating income and corporate and other costs to our consolidated results, see Note 16–Segment Information to the consolidated financial statements included elsewhere in this Annual Report on Form 10-K.
Quarterly Results of Operations
The following table sets forth selected unaudited quarterly financial data for our last seven completed fiscal quarters (Predecessor) and for the transition periods from July 1, 2021 through October 19, 2021 (Predecessor Stub Period) and from October 20, 2021 through December 31, 2021 (Successor). The information for each of these periods reflects all adjustments that are of a normal, recurring nature and that we consider necessary for a fair presentation of our operating results for such periods. The results of operations presented should be read in conjunction with our audited consolidated financial statements and notes thereto appearing elsewhere in this document and are not necessarily indicative of our operating results for any future period. Revenues for certain quarters/periods are impacted by the capital spending patterns of government customers, which are influenced by budgetary considerations and driven by timing of fiscal year-ends.
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Successor | | | Predecessor |
| ($ in millions) | From October 20, 2021 through December 31, 2021 | | | From July 1, 2021 through October 19, 2021 | | September 30, 2021 | | June 30, 2021 | | March 31, 2021 | | December 31, 2020 | | September 30, 2020 | | June 30, 2020 | | March 31, 2020 |
| Revenues | $ | 154.1 | | | | $ | 168.0 | | | $ | 144.3 | | | $ | 180.0 | | | $ | 166.2 | | | $ | 150.8 | | | $ | 114.6 | | | $ | 141.2 | | | $ | 109.8 | |
Adjusted revenues(1)(2) | $ | 156.4 | | | | $ | 172.5 | | | $ | 148.0 | | | $ | 183.7 | | | $ | 170.5 | | | $ | 150.8 | | | $ | 114.6 | | | $ | 141.4 | | | $ | 109.8 | |
| Net loss | $ | (23.0) | | | | $ | (105.7) | | | $ | (46.7) | | | $ | (27.4) | | | $ | (71.4) | | | $ | (19.2) | | | $ | (40.4) | | | $ | (24.5) | | | $ | (36.4) | |
Adjusted net income (loss)(1)(3) | $ | 25.6 | | | | $ | (33.9) | | | $ | (20.1) | | | $ | 3.2 | | | $ | (40.7) | | | $ | 3.7 | | | $ | (20.9) | | | $ | (5.4) | | | $ | (24.7) | |
| Net loss per common share attributable to Mirion Technologies, Inc. (Successor) | $ | (0.12) | | | | N/A | | N/A | | N/A | | N/A | | N/A | | N/A | | N/A | | N/A |
Adjusted EPS(1)(4) | $ | 0.14 | | | | N/A | | N/A | | N/A | | N/A | | N/A | | N/A | | N/A | | N/A |
EBITA(1)(5) | $ | 8.4 | | | | $ | (38.8) | | | $ | 8.5 | | | $ | 22.7 | | | $ | 13.8 | | | $ | 16.4 | | | $ | 8.8 | | | $ | 25.9 | | | $ | 17.9 | |
EBITDA(1)(5) | $ | 13.7 | | | | $ | (32.6) | | | $ | 13.6 | | | $ | 29.7 | | | $ | 18.8 | | | $ | 21.0 | | | $ | 13.1 | | | $ | 30.4 | | | $ | 22.1 | |
Adjusted EBITDA(1)(5) | $ | 44.5 | | | | $ | 31.2 | | | $ | 30.9 | | | $ | 50.0 | | | $ | 39.8 | | | $ | 38.3 | | | $ | 24.1 | | | $ | 40.9 | | | $ | 25.0 | |
(1)Adjusted revenues, Adjusted net (loss) income, Adjusted EPS, EBITA, EBITDA and Adjusted EBITDA are supplemental measures of our performance that are not required by, or presented in accordance with, U.S. GAAP. Adjusted revenues, Adjusted net (loss) income, Adjusted EPS, EBITA, EBITDA, and Adjusted EBITDA are included in this document because they are key metrics used by management to assess our financial performance. We believe that these measures are useful because they provide investors with information regarding our operating performance that is used by our management in its reporting and planning processes. These measures may not be comparable to similarly titled measures and disclosures reported by other companies.
Adjusted revenues are defined as U.S. GAAP revenues adjusted to remove the impact of purchase accounting on the recognition of deferred revenue. We have acquired businesses whose net tangible assets include deferred revenue. In accordance with GAAP reporting requirements, we recorded adjustments reducing deferred revenue under arrangements predating the business combination to fair value for all business combinations occurring prior. Therefore, our GAAP revenues after the date of acquisition will not reflect the full amount of revenues that would have been reported if the acquired deferred revenue was not written down to fair value. Therefore, Adjusted revenues reverses the impact of this deferred revenue write-down to provide another view of the revenue run-rate in a given period and providing meaningful information for comparative results in future periods.
Adjusted net (loss) income is defined as U.S. GAAP net income adjusted for foreign currency gains and losses, amortization of acquired intangible assets, the impact of purchase accounting on the recognition of deferred revenue, changes in the fair value of warrants, certain non-operating expenses (certain purchase accounting impacts related to revenues and inventory, restructuring and costs to achieve operational synergies, merger and acquisition expenses and IT project implementation expenses), and income tax impacts of these adjustments. Adjusted EPS is defined as adjusted net (loss) income divided by weighted average common shares outstanding — basic and diluted.
EBITA is defined as income before net interest expenses (including loss on debt extinguishment), income tax (benefit) provision, and amortization. EBITDA is defined as income before net interest expense (including loss on debt extinguishment), income tax (benefit) provision, and depreciation and amortization. EBITA and EBITDA are not terms defined under U.S. GAAP and do not purport to be alternatives to net income as measures of operating performance or to cash flows from operating activities as measures of liquidity. Additionally, EBITA and EBITDA are not intended to be measures of free cash flow available for management’s discretionary use as they do not consider certain cash requirements such as interest payments, tax payments and debt service requirements.
Adjusted EBITDA is defined as EBITDA excluding the items described in the table below. Adjusted EBITDA is used by management as a measure of operating performance. We believe that the inclusion of supplementary adjustments to EBITDA applied in presenting Adjusted EBITDA is appropriate to provide additional information to investors about our results of operations that management utilizes on an ongoing basis to assess our core operating performance.
EBITA, EBITDA and Adjusted EBITDA may not be comparable to similarly titled measures used by other companies. You should not consider our EBITA, EBITDA and Adjusted EBITDA as alternatives to operating income or net income, determined in accordance with U.S. GAAP.
(2)The following table reconciles Adjusted revenues to the most directly comparable U.S. GAAP financial performance measure, which is revenues:
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Successor | | | Predecessor |
| ($ in millions) | From October 20, 2021 through December 31, 2021 | | | From July 1, 2021 through October 19, 2021 | | September 30, 2021 | | June 30, 2021 | | March 31, 2021 | | December 31, 2020 | | September 30, 2020 | | June 30, 2020 | | March 31, 2020 |
| Revenues | $ | 154.1 | | | | $ | 168.0 | | | $ | 144.3 | | | $ | 180.0 | | | $ | 166.2 | | | $ | 150.8 | | | $ | 114.6 | | | $ | 141.2 | | | $ | 109.8 | |
| Revenue reduction from purchase accounting | 2.3 | | | | 4.5 | | | 3.7 | | | 3.7 | | | 4.3 | | | — | | | — | | | 0.2 | | | — | |
| Adjusted revenues | $ | 156.4 | | | | $ | 172.5 | | | $ | 148.0 | | | $ | 183.7 | | | $ | 170.5 | | | $ | 150.8 | | | $ | 114.6 | | | $ | 141.4 | | | $ | 109.8 | |
(3)The following table reconciles Adjusted net income (loss) to the most directly comparable U.S. GAAP financial performance measure, which is net loss:
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Successor | | | Predecessor |
| ($ in millions) | From October 20, 2021 through December 31, 2021 | | | From July 1, 2021 through October 19, 2021 | | September 30, 2021 | | June 30, 2021 | | March 31, 2021 | | December 31, 2020 | | September 30, 2020 | | June 30, 2020 | | March 31, 2020 |
| Net loss | $ | (23.0) | | | | $ | (105.7) | | | $ | (46.7) | | | $ | (27.4) | | | $ | (71.4) | | | $ | (19.2) | | | $ | (40.4) | | | $ | (24.5) | | | $ | (36.4) | |
| Revenue reduction from purchase accounting | 2.3 | | | | 4.5 | | | 3.7 | | | 3.7 | | | 4.3 | | | — | | | — | | | 0.2 | | | — | |
| Cost of revenues impact from inventory valuation purchase accounting | 15.8 | | | | — | | | — | | | — | | | 4.7 | | | 0.5 | | | — | | | 0.5 | | | 0.5 | |
| Foreign currency loss (gain), net | 1.6 | | | | (0.6) | | | (1.4) | | | 1.1 | | | (4.0) | | | 8.2 | | | 8.1 | | | 3.4 | | | (2.0) | |
| Amortization of acquired intangibles | 32.0 | | | | 19.7 | | | 16.1 | | | 18.6 | | | 18.6 | | | 13.5 | | | 12.2 | | | 12.4 | | | 12.7 | |
| Stock based compensation | 5.3 | | | | 9.3 | | | — | | | — | | | (0.1) | | | 0.1 | | | — | | | — | | | 0.1 | |
| Change in fair value of warrant liabilities | (1.2) | | | | — | | | — | | | — | | | — | | | — | | | — | | | — | | | — | |
| Debt extinguishment | — | | | | 15.9 | | | — | | | — | | | — | | | — | | | — | | | — | | | — | |
| Non-operating expenses | 7.0 | | | | 34.7 | | | 15.0 | | | 15.6 | | | 16.1 | | | 8.5 | | | 2.9 | | | 6.4 | | | 4.3 | |
| Tax impact of adjustments above | (14.2) | | | | (11.7) | | | (6.8) | | | (8.4) | | | (9.0) | | | (7.8) | | | (3.7) | | | (3.8) | | | (3.8) | |
| Adjusted net income (loss) | $ | 25.6 | | | | $ | (33.9) | | | $ | (20.1) | | | $ | 3.2 | | | $ | (40.8) | | | $ | 3.8 | | | $ | (20.9) | | | $ | (5.4) | | | $ | (24.6) | |
| Weighted average common shares outstanding — basic and diluted | 180.773 | | | | N/A | | N/A | | N/A | | N/A | | N/A | | N/A | | N/A | | N/A |
| Adjusted EPS | $ | 0.14 | | | | N/A | | N/A | | N/A | | N/A | | N/A | | N/A | | N/A | | N/A |
(4)The following table reconciles Adjusted EPS to the most directly comparable U.S. GAAP financial performance measure, which is Net loss per common share attributable to Mirion Technologies, Inc. (Successor):
| | | | | |
| Successor* |
| (Amounts per share, except for outstanding shares) | From October 20, 2021 through December 31,
2021 |
| Net loss per common share attributable to Mirion Technologies, Inc. (Successor) | $ | (0.12) | |
| Loss attributable to noncontrolling interests | (0.01) | |
| Revenue reduction from purchase accounting | 0.01 | |
| Cost of revenues impact from inventory valuation purchase accounting | 0.09 | |
| Foreign currency loss (gain), net | 0.01 | |
| Amortization of acquired intangibles | 0.18 | |
| Stock based compensation | 0.03 | |
| Change in fair value of warrant liabilities | (0.01) | |
| Non-operating expenses | 0.04 | |
| Tax impact of adjustments above | (0.08) | |
| Adjusted EPS | $ | 0.14 | |
| |
| Weighted average common shares outstanding — basic and diluted | 180.773 | |
| Dilutive Potential Common Shares - RSU's | 0.003 | |
| Adjusted weighted average common shares — diluted | 180.776 | |
* Note that Predecessor quarters have not been presented as Adjusted EPS is not meaningful for periods prior to the Business Combination due to the change in the capital structure.
(5)The following table reconciles EBITA, EBITDA and Adjusted EBITDA to the most directly comparable U.S. GAAP financial performance measure, which is net loss:
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Successor | | | Predecessor |
| ($ in millions) | From October 20, 2021 through December 31, 2021 | | | From July 1, 2021 through October 19,
2021 | | September 30, 2021 | | June 30, 2021 | | March 31, 2021 | | December 31, 2020 | | September 30, 2020 | | June 30, 2020 | | March 31, 2020 |
| Net loss | $ | (23.0) | | | | $ | (105.7) | | | $ | (46.7) | | | $ | (27.4) | | | $ | (71.4) | | | $ | (19.2) | | | $ | (40.4) | | | $ | (24.5) | | | $ | (36.4) | |
| Interest expense, net | 6.2 | | | | 52.8 | | | 43.8 | | | 43.7 | | | 43.0 | | | 38.5 | | | 38.0 | | | 38.7 | | | 39.2 | |
| Income tax (benefit) provision | (6.8) | | | | (5.6) | | | (4.7) | | | (12.1) | | | 23.6 | | | (16.4) | | | (1.0) | | | (0.7) | | | 2.4 | |
| Amortization | 32.0 | | | | 19.7 | | | 16.1 | | | 18.5 | | | 18.6 | | | 13.5 | | | 12.2 | | | 12.4 | | | 12.7 | |
| EBITA | $ | 8.4 | | | | $ | (38.8) | | | $ | 8.5 | | | $ | 22.7 | | | $ | 13.8 | | | $ | 16.4 | | | $ | 8.8 | | | $ | 25.9 | | | $ | 17.9 | |
| Depreciation | 5.3 | | | | 6.2 | | | 5.1 | | | 6.9 | | | 5.0 | | | 4.6 | | | 4.3 | | | 4.5 | | | 4.2 | |
| EBITDA | $ | 13.7 | | | | $ | (32.6) | | | $ | 13.6 | | | $ | 29.6 | | | $ | 18.8 | | | $ | 21.0 | | | $ | 13.1 | | | $ | 30.4 | | | $ | 22.1 | |
| Stock compensation expense | 5.3 | | | | 9.3 | | | — | | | — | | | (0.1) | | | 0.1 | | | — | | | — | | | 0.1 | |
| Change in fair value of warrant liabilities | (1.2) | | | | — | | | — | | | | | | | | | | | | | |
| Debt extinguishment | — | | | | 15.9 | | | — | | | — | | | — | | | — | | | — | | | — | | | — | |
| Foreign currency loss (gain), net | 1.6 | | | | (0.6) | | | (1.4) | | | 1.1 | | | (4.0) | | | 8.2 | | | 8.1 | | | 3.4 | | | (2.0) | |
| Revenue reduction from purchase accounting | 2.3 | | | | 4.5 | | | 3.7 | | | 3.7 | | | 4.3 | | | — | | | — | | | 0.2 | | | — | |
| Cost of revenues impact from inventory valuation purchase accounting | 15.8 | | | | — | | | — | | | — | | | 4.7 | | | 0.5 | | | — | | | 0.5 | | | 0.5 | |
| Non-operating expenses | 7.0 | | | | 34.7 | | | 15.0 | | | 15.6 | | | 16.1 | | | 8.5 | | | 2.9 | | | 6.4 | | | 4.3 | |
| Adjusted EBITDA | $ | 44.5 | | | | $ | 31.2 | | | $ | 30.9 | | | $ | 50.0 | | | $ | 39.8 | | | $ | 38.3 | | | $ | 24.1 | | | $ | 40.9 | | | $ | 25.0 | |
Liquidity and Capital Resources
Overview of Liquidity
Our primary future cash needs relate to working capital, operating activities, capital spending, strategic investments, and debt service.
Mirion management believes that net cash provided by operating activities, augmented by long-term debt arrangements, will provide adequate liquidity for the next 12 months of independent operations, as well as the resources necessary to invest for growth in existing businesses and manage its capital structure on a short- and long-term basis. Access to capital and availability of financing on acceptable terms in the future will be affected by many factors, including our credit rating, economic conditions, and the overall liquidity of capital markets. There can be no assurance of continued access to capital markets on acceptable terms.
At December 31, 2021, June 30, 2021, and June 30, 2020, we had $84.0 million, $101.1 million and $118.4 million, respectively, in cash and cash equivalents, which include amounts held by entities outside of the United States of approximately $69.5 million, $67.3 million and $73.7 million, respectively, primarily in Europe and Canada. Non-U.S. cash is generally available for repatriation without legal restrictions, subject to certain taxes, mainly withholding taxes. We are asserting indefinite reinvestment of cash for non-U.S. subsidiaries. The Company has alternative repatriation options
other than dividends should the need arise. The 2021 Credit Agreement provides for up to $90.0 million of revolving borrowings.
There is a discussion in Note 8, Borrowings, of the consolidated financial statements included elsewhere in this Annual Report on Form 10-K of the long-term debt arrangements issued by Mirion. For more information on our lease commitments, See Note 9, Leased Assets, of the consolidated financial statements and for other commitments and contingencies, see Note 10, Commitments and Contingencies of the consolidated financial statements.
Debt Profile
Third Party Debt Before the Business Combination
In March 2019, Mirion Technologies (HoldingRep), Ltd., a wholly owned subsidiary of the Company, and its subsidiaries entered into a credit agreement with Morgan Stanley Senior Funding Inc., as administrative agent, certain other revolving lenders and a syndicate of institutional lenders (the “2019 Credit Facility”). The 2019 Credit Facility originally provided for financing of a $450.0 million senior secured term loan facility and a €125.0 million senior secured term loan facility, as well as a $90.0 million revolving line of credit. The 2019 Credit Facility was amended to provide an additional $34.0 million, $66.0 million and $225.0 million of senior secured term loans in July 2019, December 2019 and December 2020, respectively.
The 2019 Credit Facility was repaid in full upon the consummation of the Business Combination and replaced with the Credit Facility (as defined below).
2021 Credit Agreement
On the Closing Date, certain subsidiaries of the Company entered into a credit agreement (the “2021 Credit Agreement”) among Mirion Technologies (HoldingSub2), Ltd., a limited liability company incorporated in England and Wales, as Holdings, Mirion Technologies (US Holdings), Inc., as the Parent Borrower, Mirion Technologies (US), Inc., as the Subsidiary Borrower, the lending institutions party thereto, Citibank, N.A., as the Administrative Agent and Collateral Agent and Goldman Sachs Lending Partners, Citigroup Global Markets Inc., Jefferies Finance LLC and JPMorgan Chase Bank, N.A., as the Joint Lead Arrangers and Bookrunners.
The 2021 Credit Agreement provides for an $830 million senior secured first lien term loan facility and a $90 million senior secured revolving facility (collectively, the “Credit Facilities”). The Credit Facilities are permitted to be used to effect the Transactions (as defined in the 2021 Credit Agreement), refinance the 2019 Credit Facility referred to above and for general corporate purposes. The term loan facility is scheduled to mature on October 20, 2028 and the revolving facility is scheduled to expire and mature on October 20, 2026. The agreement requires the payment of a commitment fee of 0.50% per annum for unused revolving commitments, subject to stepdowns to 0.375% per annum and 0.25% per annum upon the achievement of specified leverage ratios. Any outstanding letters of credit issued under the 2021 Credit Agreement reduce the availability under the revolving line of credit.
The 2021 Credit Agreement is secured by a first priority lien on the equity interests of the Parent Borrower owned by Holdings and substantially all of the assets (subject to customary exceptions) of the borrowers and the other guarantors thereunder. Interest with respect to the facilities is based on, at the option of the borrowers, (i) a customary base rate formula for borrowings in U.S. dollars or (ii) a floating rate formula based on LIBOR (with customary fallback provisions) for borrowings in U.S. dollars, a floating rate formula based on EURIBOR for borrowings in Euro or a floating rate formula based on SONIA for borrowings in Pounds Sterling, each as described in the 2021 Credit Agreement with respect to the applicable type of borrowing.
The 2021 Credit Agreement contains customary representations and warranties as well as customary affirmative and negative covenants and events of default. The negative covenants include, among others and in each case subject to certain thresholds and exceptions, limitations on incurrence of liens, limitations on incurrence of indebtedness, limitations on making dividends and other distributions, limitations on engaging in asset sales, limitations on making investments, and a financial covenant that the “First Lien Net Leverage Ratio” (as defined in the 2021 Credit Agreement) as of the end of any fiscal quarter is not greater than 7.00 to 1.00 if on the last day of such fiscal quarter certain borrowings outstanding under the revolving credit facility exceed 40% of the total revolving credit commitments at such time. The covenants also contain limitations on the activities of Mirion Technologies (HoldingSub2), Ltd. as the “passive” holding company. If any of the events of default occur and are not cured or waived, any unpaid amounts under the 2021 Credit Agreement may be declared immediately due and payable, the revolving credit commitments may be terminated and remedies against the collateral may be exercised.
Cash flows
Transition periods from October 20, 2021 through December 31, 2021 (Successor) and July 1, 2021 through October 19, 2021 (Predecessor Stub Period) Compared to Six Months Ended December 31, 2020 (Predecessor)
| | | | | | | | | | | | | | | | | | | | |
| Successor | | | Predecessor | | |
| | From October 20, 2021
through December 31, 2021 | | | From July 1, 2021
through
October 19, 2021 | | Six Months Ended December 31, 2020 (unaudited) |
| Net cash (used in) provided by operating activities | $ | (12.2) | | | | $ | 13.1 | | | $ | 19.4 | |
| Net cash used in investing activities | $ | (2,189.4) | | | | $ | (12.5) | | | $ | (284.5) | |
| Net cash provided by financing activities | $ | 1,537.7 | | | | $ | 1.0 | | | $ | 249.3 | |
Non-GAAP:
| | | | | | | | | | | | | | | | | | | | |
| Successor | | | Predecessor | | |
| (Dollars in millions) | From
October 20, 2021
through
December 31, 2021 | | | From July 1, 2021 through
October 19, 2021 | | Six Months Ended December 31, 2020 (unaudited) |
| Net cash provided by operating activities | $ | (12.2) | | | | $ | 13.1 | | | $ | 19.4 | |
| Purchases of property, plant, and equipment and badges | (6.0) | | | | (11.6) | | | (9.3) | |
Free cash flow(1) | $ | (18.2) | | | | $ | 1.5 | | | $ | 10.1 | |
| Cash used for non-operating expenses | 43.6 | | | | 13.4 | | | 11.1 | |
Adjusted free cash flow(1) | $ | 25.4 | | | | $ | 14.9 | | | $ | 21.2 | |
(1)Free cash flow and Adjusted free cash flow are supplemental measures of our performance that are not required by, or presented in accordance with, U.S. GAAP. We believe that Free cash flow and Adjusted free cash flow are important because they provide management with measurements of cash generated from operations that is available for payment obligations and investment opportunities, such as repaying debt and funding acquisitions.
Free cash flow is defined as U.S. GAAP net cash provided by operating activities adjusted to include the impact of purchases of property, plant, and equipment and purchases of badges. Adjusted free cash flow is defined as Free cash flow adjusted to include the impact of cash used to fund non-operating expenses (as previously defined). We believe that the inclusion of supplementary adjustments to Free cash flow applied in presenting Adjusted free cash flow is appropriate to provide additional information to investors about our cash flows that management utilizes on an ongoing basis to assess our ability to generate cash for use in acquisitions and other investing and financing activities.
Free cash flow and Adjusted free cash flow may not be comparable to similarly titled measures used by other companies. You should not consider our Free cash flow or Adjusted free cash flow as alternatives to net cash provided by (used for) operating activities in accordance with U.S. GAAP.
Net Cash Provided by Operating Activities
Net cash (used in) or provided by operating activities was $(12.2) million for the Successor period and $13.1 million for the Predecessor Stub Period which was a decrease of $18.5 million over the net cash provided by operating activities of $19.4 million for the unaudited six months ended December 31, 2020.
The decrease compared to the prior year unaudited comparable period is primarily due to a decrease in cash inflows of $15.9 million resulting from net loss adjusted for non-cash items, which was predominantly driven by Business Combination transaction fees recorded in the Predecessor Stub Period. Cash from working capital was flat, comparing the Successor and Predecessor Stub Period with the unaudited six months ended December 31, 2020. Within working capital, accounts receivable increased by $31.9 million as a result of higher billings, accrued expense decreased $1.9 million and net other assets and liabilities increased $2.9 million. These working capital cash outflows were mostly offset by an
increase in accounts payable of $14.0 million, a decrease in inventory of $1.2 million from higher sales and an increase in net, deferred contract revenue and associated deferred costs of $15.2 million.
Net Cash Used in Investing Activities
Net cash used in investing activities was $2.2 billion in the Successor period and $12.5 million in the Predecessor Stub Period. The periods reflected the Business Combination of $2.1 billion, acquisitions of $59.5 million, primarily related to CIRS of $54 million, and capital expenditures of $6.0 million and $11.6 million related to property, plant, and equipment and badges in the Successor Period and the Predecessor Stub Period, respectively.
Net Cash Provided by Financing Activities
Net cash provided by financing activities was $1.5 billion in the Successor period and was immaterial in the Predecessor Stub Period. Cash inflow in the Successor Period related to the Business Combination including $807.3 million of new borrowings, $753.7 million from issuance of stock, net of redemptions, $18.7 million of transaction fees reimbursed by the Sellers. These cash inflows were partially offset by $26.3 million in deferred underwriting fees and $13.3 million of stock issuance costs.
Fiscal year ended June 30, 2021 compared to fiscal year ended June 30, 2020
| | | | | | | | | | | | | | | | | | | | | | | |
| (Dollars in millions) | 2021 | | 2020 | | $ Change | | % Change |
| Net cash provided by operating activities | $ | 53.6 | | | $ | 39.5 | | | $ | 14.1 | | | 35.7 | % |
| Net cash used in investing activities | $ | (313.3) | | | $ | (75.6) | | | $ | (237.7) | | | 314.4 | % |
| Net cash provided by financing activities | $ | 239.0 | | | $ | 118.9 | | | $ | 120.1 | | | 101.0 | % |
Non-GAAP:
| | | | | | | | | | | |
| (Dollars in millions) | 2021 | | 2020 |
| Net cash provided by operating activities | $ | 53.6 | | | $ | 39.5 | |
| Purchases of property, plant, equipment and badges | (23.2) | | | (19.9) | |
| Free cash flow | $ | 30.4 | | | $ | 19.6 | |
| Cash used for non-operating expenses | 30.8 | | | 16.4 | |
| Adjusted free cash flow | $ | 61.2 | | | $ | 36.0 | |
Net Cash Provided by Operating Activities
Net cash provided by operating activities was $53.6 million during the year ended June 30, 2021, a $14.1 million, or 35.7%, increase compared to the year ended June 30, 2020 primarily due to cash inflow resulting from a decrease in net loss adjusted for non-cash items of approximately $5.9 million and cash inflow of $8.2 million from improved working capital (cash inflows of $5.1 million in accounts payable and $23.9 million from other operating assets and liabilities, offset by cash outflows of $8.0 million in accounts receivable, $3.3 million in inventories, and $9.5 million in accrued expenses and other current liabilities).
Net Cash Used in Investing Activities
Net cash used in investing activities was $313.3 million during the year ended June 30, 2021 compared to net cash used in investing activities of $75.6 million in the year ended June 30, 2020. The $237.7 million, or 314.4%, increase is primarily the result of greater acquisition activity (an increase of $234.4 million) in addition to purchases of property, plant and equipment and badges. Capital expenditures were $23.2 million and $19.9 million in the year ended June 30, 2021 and the year ended June 30, 2020, respectively, related to property, plant, and equipment and badges.
Net Cash Provided by Financing Activities
Net cash provided by financing activities was $239.0 million in the year ended June 30, 2021 compared to $118.9 million of cash provided in the year ended June 30, 2020. During the year ended June 30, 2021, we borrowed a net $218.8 million of notes payable under the 2019 Credit Facility and a net $70.0 million of borrowings from related parties, offset by $35.0
million of repayments of borrowings on the revolver term loan and $14.8 million of repayments of principal ($8.8 million under the 2019 Credit Facility and $6.0 million of the NRG loan). During the year ended June 30, 2020, we borrowed a net $98.8 million of notes payable under the 2019 Credit Facility and $80.0 million of borrowings under the revolver, offset by $13.4 million principal repayments of notes payable, $45.0 million repayments of borrowings on the revolver term loan, $2.0 million of contingent consideration payments, and $0.4 million of distributions to noncontrolling interests.
Year ended June 30, 2020 compared to year ended June 30, 2019
| | | | | | | | | | | | | | | | | | | | | | | |
| (Dollars in millions) | 2020 | | 2019 | | $ Change | | % Change |
| Net cash provided by operating activities | $ | 39.5 | | | $ | 14.7 | | | $ | 24.8 | | | 168.7 | % |
| Net cash used in investing activities | $ | (75.6) | | | $ | (25.6) | | | $ | (50.0) | | | 195.3 | % |
| Net cash provided by financing activities | $ | 118.9 | | | $ | 15.0 | | | $ | 103.9 | | | 692.7 | % |
Non-GAAP:
| | | | | | | | | | | |
| (Dollars in millions) | 2020 | | 2019 |
| Net cash provided by operating activities | $ | 39.5 | | | $ | 14.7 | |
| Purchases of property, plant, equipment and badges | (19.9) | | | (16.5) | |
| Free cash flow | $ | 19.6 | | | $ | (1.8) | |
| Cash used for non-operating expenses | 16.4 | | | 10.3 | |
| Adjusted free cash flow | $ | 36.0 | | | $ | 8.5 | |
Net Cash Provided by Operating Activities
Net cash from operating activities was $39.5 million in FY 2020, a $24.8 million, or 168.7%, increase compared to FY 2019 resulting from a decrease in net loss adjusted for non-cash items of approximately $3.2 million offset by cash inflow of $28.0 million from improved working capital (cash inflows of $12.1 million from inventories, $0.2 million from accounts payable, $20.4 million from accrued expenses and other current liabilities, and $2.1 million of other operating assets and liabilities, offset by cash outflows of $6.8 million from accounts receivable).
Net Cash Used in Investing Activities
Net cash used in investing activities was $75.6 million in FY 2020 compared to net cash used in investing activities of $25.6 million in FY 2019. The $50.0 million, or 195.3%, increase is primarily the result of greater acquisition activity (an increase of $46.6 million) in addition to purchases of property, plant and equipment and badges. Capital expenditures were $19.9 million and $16.5 million in FY 2020 and FY 2019, respectively, related to property, plant, and equipment.
Net Cash Provided by Financing Activities
Net cash provided by financing activities was $118.9 million in FY 2020 compared to net cash provided by financing activities of $15.0 million in FY 2019. During FY 2020, we borrowed $98.8 million of notes payable (primarily under the 2019 Credit Facility) and $80.0 million of borrowings under the revolver, offset by $13.4 million principal repayments of notes payable, $45.0 million repayments of borrowings on the revolver term loan, $2.0 million of contingent consideration payments, and $0.4 million of distributions to noncontrolling interests. During FY 2019, net cash provided by financing activities was driven primarily by net borrowings due to the refinancing of debt and issuance of notes payable under the 2019 Credit Facility of $28.5 million offset by repayments of $13.0 million for the revolver.
Critical Accounting Policies and Estimates
Our consolidated financial statements are prepared in accordance with U.S. GAAP. The preparation of these financial statements requires management to make estimates and assumptions that affect the reported amounts of assets, liabilities, revenues, costs and expenses and related disclosures. Such estimates are based on historical experience and on various other factors that management believes are reasonable under the circumstances, the results of which form the basis for making judgments about the carrying value of assets and liabilities that are not readily apparent from other sources. Actual results may differ from these judgments and estimates under different assumptions or conditions and any such differences
may be material. We believe that the accounting policies discussed below are critical to understanding historical and future performance, as these policies relate to the more significant areas involving management’s judgments and estimates.
Business combinations
We account for business acquisitions in accordance with the Financial Accounting Standards Board (“FASB”) Accounting Standards Codification (“ASC”) 805, "Business Combinations". This standard requires the acquiring entity in a business combination to recognize all the assets acquired and liabilities assumed in the transaction and establishes the acquisition date fair value as the measurement objective for all assets acquired and liabilities assumed in a business combination. Certain provisions of this standard prescribe, among other things, the determination of acquisition date fair value of consideration paid in a business combination (including contingent consideration) and the exclusion of transaction and acquisition-related restructuring costs from acquisition accounting.
The determination of the fair value of assets acquired and liabilities assumed involves assessments of factors such as the expected future cash flows associated with individual assets and liabilities and appropriate discount rates at the closing date of the acquisition. For non-observable market values, the Company determines fair value using acceptable valuation principles (e.g., multiple excess earnings, relief from royalty and cost methods).
Goodwill
Goodwill represents the excess of the purchase price paid over the estimated fair value of the net assets acquired and liabilities assumed in the acquisition of a business.
Goodwill has an indefinite useful life, and is not amortized, but instead tested for impairment annually during the fiscal year fourth quarter or more often if events or changes in circumstances indicate that the carrying amount may exceed fair value as set forth in ASC 350, "Intangibles—Goodwill and Other". The Company tests for goodwill impairment at the reporting unit level, which is an operating segment or one level below an operating segment. The amount of goodwill acquired in a business combination that is assigned to one or more reporting units as of the acquisition date is the excess of the purchase price of the acquired businesses (or portion thereof) included in the reporting unit, over the fair value assigned to the individual assets acquired or liabilities assumed from a market participant perspective. Goodwill is assigned to the reporting unit(s) expected to benefit from the synergies of the combination even though other assets or liabilities of the acquired entity may not be assigned to that reporting unit.
ASC 350 allows an optional qualitative assessment as part of annual impairment testing, prior to a quantitative assessment test, to determine whether it is more likely than not that the fair value of a reporting unit exceeds its carrying amount. If a qualitative assessment determines an impairment is more likely than not, the Company is required to perform a quantitative impairment test. Otherwise, no further analysis is required. Alternatively, the Company may elect to proceed directly to the quantitative impairment test.
In conducting a qualitative assessment, the Company analyzes actual and projected growth trends for net sales and margin for each reporting unit, as well as historical performance versus plan and the results of prior quantitative tests performed. Additionally, the Company assesses factors that may impact its business, including macroeconomic conditions and the related impact, market-related exposures, plans to market for sale all or a portion of the business, competitive changes, new or discontinued product lines, changes in key personnel, and any potential risks to projected financial results.
If performed, the quantitative test compares the fair value of a reporting unit with its carrying amount. We determine the fair value of each reporting unit by estimating the present value of expected future cash flows, discounted by the applicable discount rate, and peer company multiples. If the carrying value exceeds the fair value, the Company recognizes an impairment loss in the amount equal to the excess, not to exceed the total amount of goodwill allocated to that reporting unit.
Based upon our review and analysis, no impairments were deemed to have occurred during any of the periods presented.
Intangible Assets
Intangible assets relate to the value associated with our developed technology, customer relationships, backlog, trade names and non-compete agreements at the time of acquisition through business combinations. Definite lived intangible assets are amortized over their estimated useful lives, ranging from 1 to 16 years.
Revenue Recognition
Prior to July 1, 2019, the Company recognized revenue based on ASC 605, "Revenue Recognition", when there was persuasive evidence of an arrangement, product delivery had occurred or services had been provided, the sales price was fixed or determinable and collectability was reasonably assured. Beginning July 1, 2019, the Company recognizes revenue based on ASC 606, "Revenue from Contracts with Customers" as performance obligations are satisfied by transferring control of promised goods or service to customers in an amount that reflects the consideration to which the Company expects to be entitled in exchange for those goods or services. See Recently Adopted Accounting Guidance below for a discussion of the change in revenue recognition accounting that became effective on July 1, 2019.
ASC 606
Performance Obligations
The Company identifies a performance obligation for each promise in a contract to transfer a distinct good or service to the customer. As part of its assessment, the Company considers all goods and/or services promised in the contract, regardless of whether they are explicitly stated or implied by customary business practices. The Company’s contracts may contain either a single performance obligation, including the promise to transfer individual goods or services that are not separately distinct within the context of the respective contracts, or multiple performance obligations. For contracts that contain multiple performance obligations, the Company allocates the consideration to which it expects to be entitled to each performance obligation based on relative standalone selling prices and recognizes the related revenue when or as control of each individual performance obligation is transferred to customers. Service revenues (warranty contracts, post contract support, and subscription-based services) are recognized over time as the customers receive and consume benefits of such services simultaneously.
The Company exercises judgment in determining the timing of revenue by analyzing the point in time or the period over which the customer has the ability to direct the use of and obtain substantially all of the remaining benefits of the performance obligation. Typically, over-time revenue recognition is based on the utilization of an input measure used to measure progress, such as costs incurred to date relative to total estimated costs. Changes in total estimated costs are recognized using the cumulative catch-up method of accounting which recognize the cumulative effect of the changes on current and prior periods in the current period. Accordingly, the effect of the changes on future periods of contract performance is recognized as if the revised estimate had been the original estimate. A significant change in an estimate on one or more contracts could have a material effect on the Company’s consolidated financial position, results from operations, or cash flows. However, there were no significant changes in estimated contract costs for the Successor Period of October 20, 2021 through December 31, 2021 and the Predecessor Stub Period of July 1, 2021 through October 19, 2021 and the Predecessor Period for the fiscal year ended June 30, 2021.
If a performance obligation does not qualify for over-time revenue recognition, revenue is then recognized at the point-in-time in which control of the distinct good or service is transferred to the customer, typically based upon the terms of delivery.
Certain of the Company’s products are sold through distributors and third-party sales representatives under standard agreements whereby distributors purchase products from the Company and resell them to customers. These agreements give distributors the right to sell the Company’s products within certain territories and establish minimum order requirements. These arrangements do not provide stock rotation or price protection rights and do not contain extended payment terms. Rights of return are limited to repair or replacement of delivered products that are defective or fail to meet the Company’s published specifications. Provisions for these warranty costs are recognized in the same period that the related revenue is recorded.
The remaining performance obligation for open contracts as of December 31, 2021 include assembly, delivery, installation and training. Payment terms for shipments to end-users are generally net 30 days. Payment terms for distributor shipments may range from 30 to 90 days. Service arrangements can call for payments in advance of performing the work (e.g., extended warranty and service contracts), upon completion of contract milestones (e.g., custom development manufacturing), or a combination of each.
ASC 605
Prior to July 1, 2019, the Company recognized revenue from sales contracts when there was persuasive evidence of an arrangement, product delivery had occurred or services had been provided, the sales price was fixed or determinable and
collectability was reasonably assured. For sales contracts that contain customer-specific acceptance provisions, revenue and the related costs were deferred until the customer had indicated successful completion of site acceptance tests or the Company had otherwise determined that all customer-specific acceptance criteria had been met. Where the Company performed detailed factory acceptance testing on completed products, which, in some instances, was sufficiently extensive and reliable to demonstrate that its products meet the customer-specified objective acceptance criteria set forth in the related sales arrangements. In such instances, the Company recognized revenue based on delivery terms and prior to the receipt of notification of formal acceptance from the customer.
The Company combined a group of contracts as one project if they are closely related and were in substance, part of a single project with an overall project margin. The Company segmented a contract into several projects when they were of different business substance, for example, with different business negotiation, solutions, implementation plans, and margins.
The Company evaluated each deliverable in an arrangement to determine whether they represented separate units of accounting. A deliverable constituted a separate unit of accounting when it had stand-alone value, and for an arrangement that included a general right of return relative to the delivered products or services, when delivery or performance of the undelivered product or service was considered probable and is substantially controlled by the Company. The Company considers a deliverable to have stand-alone value if the product or service is sold separately by the Company or another vendor or could be resold by the customer on a stand-alone basis at an amount that would substantially recover the original purchase price. Further, the revenue arrangements generally do not include a general right of return relative to the delivered products. Where the aforementioned criteria for a separate unit of accounting are not met, the deliverable is combined with the undelivered element(s) and treated as a single unit of accounting for the purposes of allocation of the arrangement consideration and revenue recognition.
When a sales arrangement contains multiple units of accounting, the Company allocates the total arrangement consideration to each separable element of an arrangement based upon the relative selling price of each element. Allocation of the consideration is determined at arrangement inception based on each unit’s relative selling price, which is determined based on a selling price hierarchy. The relative selling price for a deliverable is based on its vendor-specific objective evidence (“VSOE”) if available, third-party evidence (“TPE”) if VSOE is not available or estimated selling price if neither VSOE nor TPE is available. The Company then recognizes revenue on each deliverable in accordance with its policies for product and service revenue recognition. The Company is not typically able to determine VSOE or TPE and therefore uses estimated selling prices to allocate revenue between the elements of the arrangement. The Company establishes its best estimate of the selling price considering multiple factors, including, but not limited to, pricing practices in different geographies and through different sales channels, costs and margin objectives, competitive pricing strategies and general market conditions.
The Company limits the amount of revenue recognition for delivered elements to the amount that is not contingent on the future delivery of products or services or future performance obligations or subject to customer-specific cancellation rights.
For all arrangements, amounts billed to a customer related to shipping and handling are classified as revenue while all costs incurred by the Company for shipping and handling are classified as cost of revenue. Provisions and allowances for discounts to customers, estimated sales returns, service cancellations, and other adjustments are provided for in the same period that the related revenue is recorded.
Certain of the Company’s products are sold through distributors and third-party sales representatives under standard agreements whereby distributors purchase products from the Company and resell them to customers. These agreements give distributors the right to sell the Company’s products within certain territories and establish minimum order requirements. These arrangements do not provide stock rotation or price protection rights and do not contain extended payment terms. Rights of return are limited to repair or replacement of delivered products that are defective or fail to meet the Company’s published specifications. Provisions for these warranty costs are recognized in the same period that the related revenue is recorded.
Revenue from certain fixed-price contracts that involve customization of equipment to customer specifications is recorded using a percentage-of-completion method measured on the cost-to-cost basis. Contract costs include all direct materials and labor costs, as well as indirect costs related to contract performance. Changes in job performance, job conditions, and estimated profitability result in revisions to costs and revenue and are recognized in the period in which the revisions are determined. Provisions for estimated losses on uncompleted contracts are made in the period in which such losses are first determined. Revenue earned in excess of billings on contracts in progress is classified in the consolidated balance sheet as a current asset and included in costs in excess of billings on uncompleted contracts. Amounts billed in excess of revenue earned are classified as a current liability and included in deferred contract revenue.
Revenue derived from passive dosimetry and analytical services is of a subscription nature, with passive dosimetry and analytical services provided to customers on an agreed-upon recurring monthly, quarterly, or annual basis. Services are provided to the customer through passive dosimeter badges that the Company supplies to customer personnel. Depending on the type of badge utilized, either customers return the used badges to the Company for analysis, or they obtain the analysis directly through a self-service web portal. The Company believes that badge production, badge wearing, badge analysis and report preparation are all integral to the benefit that the Company provides to its customers and, therefore, the service period is defined as the period over which all of these services are provided. Revenue is recognized on a straight-line basis over the service period as the service is continuous, and no other discernible pattern of recognition is evident. Many customers pay for these measuring and monitoring services in advance. The amounts are recorded as deferred contract revenue in the consolidated balance sheets and represent customer deposits invoiced in advance for services to be rendered over the service period, net of a reserve for estimated cancellations.
Pertinent to ASC 606 and 605
The Company sells its products and services mainly to large, private, and governmental organizations in the Americas, Europe, the Middle East, and Asia Pacific regions. The Company performs ongoing evaluations of its customers’ financial condition and limits the amount of credit extended when deemed necessary. The Company generally does not require its customers to provide collateral or other security to support accounts receivable.
Accounting for Income Taxes
The Company accounts for income taxes and the related accounts under the asset and liability method. Deferred tax assets and liabilities are recognized for the expected tax consequences of temporary differences between the tax bases of assets and liabilities and their reported amounts. Valuation allowances are recorded to reduce deferred tax assets to the amount that will more likely than not be realized. The Company classifies all deferred tax assets and liabilities, and any related valuation allowance, as non-current in the consolidated balance sheet.
The Company accounts for uncertainty in income taxes using a two-step approach to recognizing and measuring uncertain tax positions. The first step is to evaluate the tax position for recognition by determining if the weight of available evidence indicates that it is more likely than not that the position will be sustained on audit, including resolution of related appeals or litigation processes, if any. The second step is to measure the tax benefit as the largest amount that is more than 50% likely of being realized upon settlement. The Company classifies the liability for unrecognized tax benefits as current in the balance sheet, to the extent that the Company anticipates payment or receipt of cash within one year. Interest and penalties related to uncertain tax positions are recognized in the provision for income taxes.
Derivative Warrant Liabilities
We do not use derivative instruments to hedge exposures to cash flow, market, or foreign currency risks. We evaluate all of our financial instruments, including issued stock purchase warrants, to determine if such instruments are derivatives or contain features that qualify as embedded derivatives, pursuant to ASC 480 and FASB ASC Topic 815, “Derivatives and Hedging”. The classification of derivative instruments, including whether such instruments should be recorded as liabilities or as equity, is re-assessed at the end of each reporting period. In accordance with ASC Topic 825-10 “Financial Instruments”, offering costs attributable to the issuance of the derivative warrant liabilities have been allocated based on their relative fair value of total proceeds and are recognized in the statement of operations as incurred.
The Public Warrants and the Private Placement Warrants are recognized as derivative liabilities in accordance with ASC 815. Accordingly, we recognize the warrant instruments as liabilities at fair value and adjust the instruments to fair value at each reporting period. The liabilities are subject to re-measurement at each balance sheet date until exercised, and any change in fair value is recognized in our statement of operations. The fair value of the Public Warrants as of December 31, 2021 is based on observable listed prices for such warrants. As the transfer of Private Placement Warrants to anyone who is not a permitted transferee would result in the Private Warrants having substantially the same terms as the Public Warrants, we determined that the fair value of each Private Warrant is equivalent to that of each Public Warrant. The determination of the fair value of the warrant liability may be subject to change as more current information becomes available and accordingly the actual results could differ significantly. Derivative warrant liabilities are classified as non-current liabilities as their liquidation is not reasonably expected to require the use of current assets or require the creation of current liabilities.
ITEM 7A. QUANTITATIVE AND QUALITATIVE DISCLOSURES ABOUT MARKET RISK
Quantitative and Qualitative Disclosures about Market Risk
Market risk
The market risk inherent in financial statements represents the potential loss in fair value, earnings or cash flows arising from adverse changes in foreign currency exchange rates, commodity prices or interest rates. We may use derivative financial instruments like interest rate swaps to manage exposure to market risks. We do not use derivative financial instruments for trading purposes.
Foreign currency exchange rate risk
In the normal course of business, we are exposed to changes in foreign currency exchange rates due to its worldwide presence and business profile. Foreign currency exposures relate to transactions denominated in currencies that differ from the function currencies of our subsidiaries.
We derived approximately 43.7% and 40.4% of our revenues during the Successor Period from October 20, 2021 through December 31, 2021 and during the Predecessor Period from July 1, 2021 through October 19, 2021, respectively, from outside the United States through international operations, some of which were transacted in U.S. dollars. In addition, certain of our domestic operations have sales to foreign customers. Although we are impacted by the exchange rates of several currencies, our largest exposures are generally to the Euro, Canadian dollar, British Pound, and Japanese Yen. In conducting our foreign operations, we also make inter-company sales denominated in different currencies. These activities expose us to the effect of changes in foreign currency exchange rates. Flows of foreign currencies into and out of our operations are generally stable, regularly occurring and are recorded at fair market value in our financial statements.
During the Successor Period from October 20, 2021 through December 31, 2021 and the Predecessor Period from July 1, 2021 through October 19, 2021 and the fiscal years ended June 30, 2021, and June 30, 2020, the effect of a hypothetical 10% change in foreign currencies that we have exposure to compared to the U.S. dollar would have impacted our revenues by approximately $8.7 million, $9.3 million, $41.7 million, and $31.6 million respectively.
During the Successor Period from October 20, 2021 through December 31, 2021 and during the Predecessor Periods from July 1, 2021 through October 19, 2021 and the fiscal years ended June 30, 2021, and June 30, 2020, the effect of a hypothetical 1% change in exchange rates would have impacted accumulated other comprehensive income by approximately $2.5 million, $4.0 million, $4.5 million, and $5.1 million, respectively. This impact does not consider the effects of a stronger or weaker dollar on our ability to compete for export business or the overall economic activity that could exist in such an environment. Changes in foreign exchange rates could impact the price and the demand for our products such as a strengthening dollar causes exports to become more expensive to foreign customers and businesses that must pay for them in other currencies.
Interest rates risk
We are exposed to changes in interest rates primarily as a result of our long-term debt. We may from time to time use interest rate swap agreements or other hedging instruments to manage the interest rate characteristics of a portion of our outstanding debt. However, as of December 31, 2021, we did not have any active interest rate swap agreements or other hedging instruments of any value. In March 2020, we executed an interest rate cap agreement effective September 30, 2020 through March 31, 2022 for a 2% LIBOR interest rate cap on $542 million notional value. This instrument was canceled in November 2021 given our new financing in October 2021. Based on the amounts and mix of our floating rate debt at December 31, 2021, if market interest rates increase an average of 100 basis points, our year-to-date interest expense would increase by approximately $8.4 million. We determined these amounts by considering the impact of the hypothetical interest rates on our borrowing costs. This analysis does not consider the effects of changes in the level of overall economic activity that could exist in such an environment.
Inflation risk
We are experiencing inflationary pressure on our operating costs. Competition for skilled labor is acute and we have experienced increased personnel costs as a result. We also continue to face higher costs for commodities and energy used in production of our goods, as well as increased prices from suppliers for components. Freight costs for inbound shipments of materials and components, and outbound shipments of our finished goods, have increased as well. These increases are
expected to persist into 2022. Given market competition we may not be able to offset these higher costs through price increases, which may materially and adversely affect our business, results of operations and financial condition. Any price increases we may impose may lead to declines in sales volume or market share, if competitors do not similarly adjust their prices, or customers refuse to purchase at the higher prices.
ITEM 8. FINANCIAL STATEMENTS AND SUPPLEMENTARY DATA
INDEX TO CONSOLIDATED FINANCIAL STATEMENTS
| | | | | |
| |
as of December 31, 2021, June 30, 2021 and June 30, 2020 | |
for the periods ended December 31, 2021 and October 19, 2021 and the fiscal years ended June 30, 2021, June 30, 2020 and June 30, 2019 | |
for the periods ended December 31, 2021 and October 19, 2021 and the fiscal years ended June 30, 2021, June 30, 2020 and June 30, 2019 | |
for the periods ended December 31, 2021 and October 19, 2021 and the fiscal years ended June 30, 2021, June 30, 2020 and June 30, 2019 | |
for the periods ended December 31, 2021 and October 19, 2021 and the fiscal years ended June 30, 2021, June 30, 2020 and June 30, 2019 | |
for the periods ended December 31, 2021 and October 19, 2021 and the fiscal years ended June 30, 2021, June 30, 2020 and June 30, 2019 | |
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
To the Stockholders and the Board of Directors of Mirion Technologies, Inc.
Opinion on the Financial Statements
We have audited the accompanying consolidated balance sheets of Mirion Technologies, Inc. and subsidiaries (the "Company") as of December 31, 2021 (Successor), June 30, 2021 (Predecessor), and June 30, 2020 (Predecessor), the related consolidated statements of operations, comprehensive loss, stockholders' equity (deficit), and cash flows, for the period from October 20, 2021 through December 31, 2021 (Successor), the period from July 1, 2021 through October 19, 2021 (Predecessor) and the three years ended June 30, 2021 (Predecessor), and the related notes and the schedules listed in the Index at Item 15 (collectively referred to as the "financial statements"). In our opinion, the financial statements present fairly, in all material respects, the financial position of the Company as of December 31, 2021 (Successor), June 30, 2021 (Predecessor) and June 30, 2020 (Predecessor), and the results of its operations and its cash flows for the period from October 20, 2021 through December 31, 2021 (Successor), the period from July 1, 2021 through October 19, 2021 (Predecessor) and the three years ended June 30, 2021 (Predecessor), in conformity with accounting principles generally accepted in the United States of America.
Basis for Opinion
These financial statements are the responsibility of the Company's management. Our responsibility is to express an opinion on the Company's financial statements based on our audits. We are a public accounting firm registered with the Public Company Accounting Oversight Board (United States) (PCAOB) and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audits in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement, whether due to error or fraud. The Company is not required to have, nor were we engaged to perform, an audit of its internal control over financial reporting. As part of our audits, we are required to obtain an understanding of internal control over financial reporting but not for the purpose of expressing an opinion on the effectiveness of the Company’s internal control over financial reporting. Accordingly, we express no such opinion.
Our audits included performing procedures to assess the risks of material misstatement of the financial statements, whether due to error or fraud, and performing procedures that respond to those risks. Such procedures included examining, on a test basis, evidence regarding the amounts and disclosures in the financial statements. Our audits also included evaluating the
accounting principles used and significant estimates made by management, as well as evaluating the overall presentation of the financial statements. We believe that our audits provide a reasonable basis for our opinion.
Critical Audit Matters
The critical audit matters communicated below are matters arising from the current-period audit of the financial statements that were communicated or required to be communicated to the audit committee and that (1) relate to accounts or disclosures that are material to the financial statements and (2) involved our especially challenging, subjective, or complex judgments. The communication of critical audit matters does not alter in any way our opinion on the financial statements, taken as a whole, and we are not, by communicating the critical audit matters below, providing separate opinions on the critical audit matters or on the accounts or disclosures to which they relate.
Business Combinations and Acquisitions – Mirion TopCo Acquisition – Intangible Assets — Refer to Notes 1 and 2 to the financial statements
Critical Audit Matter Description
The Company completed the acquisition of Mirion Topco for $2.6 billion on October 20, 2021. The Company accounted for the acquisition under the acquisition method of accounting for business combinations. Accordingly, the purchase price was allocated to the assets acquired and liabilities assumed based on their respective fair values, including customer relationship intangible assets of $338.8 million and technology intangible assets of $234.6 million. Management estimated the fair value of the customer relationship and technology intangible assets using the multi-period excess earnings method, which is a specific discounted cash flow method. The fair value determination of the acquired intangible assets require management to make significant estimates and assumptions related to future cash flows and the selection of the discount rate.
Given the fair value determination of acquired intangible assets for Mirion Topco requires management to make significant estimates and assumptions related to the forecasts of future cash flows and the selection of the discount rate, performing audit procedures to evaluate the reasonableness of these estimates and assumptions required a high degree of auditor judgment and an increased extent of effort, including the need to involve our fair value specialists.
How the Critical Audit Matter Was Addressed in the Audit
Our audit procedures related to the forecasts of future cash flows and the selection of the discount rate for the acquired intangible assets included the following, among others:
•We assessed the reasonableness of management’s forecasts of future cash flows by comparing the projections to historical results and industry forecasts.
•With the assistance of our fair value specialists, we evaluated the reasonableness of the (1) valuation methodology and (2) discount rate by:
◦Testing the source information underlying the determination of the discount rate and testing the mathematical accuracy of the calculation.
◦Developing a range of independent estimates and comparing those to the discount rate selected by management.
•We evaluated whether the estimated future cash flows were consistent with evidence obtained in other areas of the audit.
•We evaluated management’s ability to accurately forecast future cash flows by comparing historical results to management’s forecasts.
Contracts in Progress — Refer to Notes 1 and 3 to the financial statements
Critical Audit Matter Description
The Company recognizes revenue from certain fixed-price contracts that involve customization of equipment to customer specifications using a percentage-of-completion method measured on the cost-to-cost basis, because transfer of control to the customer is continuous. The accounting for these contracts involves judgment, particularly as it relates to the process of estimating total costs for the performance obligation. The Company uses costs incurred as the input method to determine progress, and revenue is recognized based on costs incurred to date plus the estimate of the margin at completion.
Given the judgments necessary to estimate total costs to complete for certain fixed-price contracts that involve customization of equipment, auditing such estimates required extensive audit effort due to the subjectivity of cost to
complete estimates and a high degree of auditor judgment when performing audit procedures and evaluating the results of those procedures.
How the Critical Audit Matter Was Addressed in the Audit
Our audit procedures related to Contracts in Progress included the following, among others:
•We selected a sample fixed-price contracts that involve customization of equipment and performed the following:
◦Evaluated whether the contracts were properly included in management’s calculation of percentage-of-completion revenue based on the terms and conditions of each contract, including whether continuous transfer of control to the customer occurred as progress was made toward fulfilling the performance obligation.
◦Compared the transaction prices to the consideration expected to be received based on current rights and obligations under the contracts and any modifications that were agreed upon with the customers.
◦Tested management’s identification of distinct performance obligations by evaluating whether the underlying goods, services, or both were highly interdependent and interrelated.
◦Tested the accuracy and completeness of the costs incurred to date for the performance obligation.
◦Evaluated the estimates of total cost by:
▪Comparing the expected total cost to previous estimates of expected total cost to identify potential bias in estimates.
▪Evaluating management’s ability to achieve the estimates of total cost and profit by performing corroborating inquiries with the Company’s project managers and engineers.
▪Comparing management’s estimates of cost for certain labor and material inputs to salary information and vendor invoices or vendor quotes, when applicable.
◦Tested the mathematical accuracy of management’s calculation of revenue for the performance obligation.
•We evaluated management’s ability to estimate total costs accurately by evaluating significant fluctuations in margins year over year on percentage-of-completion contracts.
/s/ Deloitte & Touche LLP
Atlanta, Georgia
February 28, 2022
We have served as the Company's auditor since 2015.
Mirion Technologies, Inc.
Consolidated Balance Sheets
(In millions, except share data)
| | | | | | | | | | | | | | | | | |
| Successor | | | Predecessor |
| December 31,
2021 | | | June 30,
2021 | June 30,
2020 |
| ASSETS | | | | | |
| Current assets: | | | | | |
| Cash and cash equivalents | $ | 84.0 | | | | $ | 101.1 | | $ | 118.4 | |
| Restricted cash | 0.6 | | | | 0.8 | | 1.1 | |
| Accounts receivable, net of allowance for doubtful accounts | 157.4 | | | | 133.3 | | 97.3 | |
| Costs in excess of billings on uncompleted contracts | 56.3 | | | | 57.2 | | 59.5 | |
| Inventories | 123.6 | | | | 113.2 | | 90.2 | |
| Deferred cost of revenue | 0.6 | | | | 0.3 | | 6.5 | |
| Prepaid expenses and other currents assets | 30.9 | | | | 28.0 | | 16.7 | |
| Total current assets | 453.4 | | | | 433.9 | | 389.7 | |
| Property, plant, and equipment, net | 124.0 | | | | 88.8 | | 75.2 | |
| Operating ROU assets | 45.7 | | | | — | | — | |
| Goodwill | 1,662.6 | | | | 681.5 | | 522.6 | |
| Intangible assets, net | 806.9 | | | | 326.3 | | 248.3 | |
| Restricted cash | 0.7 | | | | 0.5 | | 0.5 | |
| Other assets | 24.7 | | | | 16.2 | | 7.5 | |
| Total assets | $ | 3,118.0 | | | | $ | 1,547.2 | | $ | 1,243.8 | |
| LIABILITIES AND STOCKHOLDERS’ EQUITY (DEFICIT) | | | | | |
| Current liabilities: | | | | | |
| Accounts payable | $ | 59.4 | | | | $ | 47.1 | | $ | 38.7 | |
| Deferred contract revenue | 73.0 | | | | 50.4 | | 39.6 | |
| Notes payable to third-parties, current | 3.9 | | | | 6.4 | | 41.1 | |
| Operating lease liability, current | 9.3 | | | | — | | — | |
| Accrued expenses and other current liabilities | 75.4 | | | | 84.3 | | 64.1 | |
| Total current liabilities | 221.0 | | | | 188.2 | | 183.5 | |
| Notes payable to related parties, non-current | — | | | | 1,170.5 | | 987.1 | |
| Notes payable to third-parties, non-current | 806.8 | | | | 885.7 | | 669.8 | |
| Warrant liabilities | 68.1 | | | | — | | — | |
| Interest accrued on notes payable to related parties | — | | | | 64.8 | | 56.4 | |
| Operating lease liability, non-current | 40.6 | | | | — | | — | |
| Deferred income taxes and other liabilities | 197.5 | | | | 77.5 | | 63.5 | |
| Total liabilities | 1,334.0 | | | | 2,386.7 | | 1,960.3 | |
| Commitments and contingencies (Note 9) | | | | | |
| Stockholders’ equity (deficit): | | | | | |
Class A common stock (Successor); $0.0001 par value, 500,000,000 shares authorized; 199,523,292 issued and outstanding at December 31, 2021 | — | | | | — | | — | |
Class B common stock (Successor); $0.0001 par value, 100,000,000 shares authorized; 8,560,540 issued and outstanding at December 31, 2021 | — | | | | — | | — | |
A Ordinary shares (Predecessor), $0.01 nominal value, 3,000,000 shares authorized, 1,483,795 issued and outstanding at June 30, 2021 and June 30, 2020 | — | | | | — | | — | |
B Ordinary shares (Predecessor), $0.01 nominal value ,7,000,000 shares authorized, 5,353,970 issued and outstanding at both June 30, 2021 and June 30, 2020 | — | | | | 0.1 | | 0.1 | |
| Additional paid-in capital | 1,845.5 | | | | 9.5 | | 9.5 | |
| Receivable from Employees for purchase of Ordinary Shares | — | | | | (2.4) | | (2.7) | |
| Accumulated deficit | (131.6) | | | | (888.0) | | (729.7) | |
| Accumulated other comprehensive (loss) income | (20.7) | | | | 39.2 | | 4.1 | |
| Mirion Technologies, Inc. (Successor) and Mirion Technologies (TopCo), Ltd. (Predecessor) stockholders’ equity (deficit) | 1,693.2 | | | | (841.6) | | (718.7) | |
| Noncontrolling interests | 90.8 | | | | 2.1 | | 2.2 | |
| Total stockholders’ equity (deficit) | 1,784.0 | | | | (839.5) | | (716.5) | |
| Total liabilities and stockholders’ equity (deficit) | $ | 3,118.0 | | | | $ | 1,547.2 | | $ | 1,243.8 | |
The accompanying notes are an integral part of these consolidated financial statements.
Mirion Technologies, Inc.
Consolidated Statements of Operations
(In millions, except per share data)
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | Successor | | | Predecessor |
| | From
October 20, 2021
through
December 31, 2021 | | | From July 1, 2021 through
October 19, 2021 | | Fiscal Year Ended June 30, 2021 | | Fiscal Year Ended June 30, 2020 | | Fiscal Year Ended June 30, 2019 |
| Revenues: | | | | | | | | | | |
| Product | $ | 120.9 | | | | $ | 123.4 | | | $ | 459.3 | | | $ | 353.0 | | | $ | 325.7 | |
| Service | 33.2 | | | | 44.6 | | | 152.3 | | | 125.2 | | | 114.4 | |
| Total revenues | 154.1 | | | | 168.0 | | | 611.6 | | | 478.2 | | | 440.1 | |
| Cost of revenues: | | | | | | | | | | |
| Product | 83.1 | | | | 74.0 | | | 284.1 | | | 216.8 | | | 190.7 | |
| Service | 17.1 | | | | 23.7 | | | 75.7 | | | 64.4 | | | 61.2 | |
| Total cost of revenues | 100.2 | | | | 97.7 | | | 359.8 | | | 281.2 | | | 251.9 | |
| Gross profit | 53.9 | | | | 70.3 | | | 251.8 | | | 197.0 | | | 188.2 | |
| Operating expenses: | | | | | | | | | | |
| Selling, general and administrative | 70.1 | | | | 101.6 | | | 211.2 | | | 158.1 | | | 145.4 | |
| Research and development | 6.7 | | | | 10.3 | | | 29.4 | | | 15.9 | | | 14.0 | |
| Total operating expenses | 76.8 | | | | 111.9 | | | 240.6 | | | 174.0 | | | 159.4 | |
| (Loss) income from operations | (22.9) | | | | (41.6) | | | 11.2 | | | 23.0 | | | 28.8 | |
| Other expense (income): | | | | | | | | | | |
| Third party interest expense | 6.2 | | | | 12.5 | | | 41.0 | | | 41.5 | | | 47.7 | |
| Related party interest expense | — | | | | 40.3 | | | 122.2 | | | 107.7 | | | 95.8 | |
| Loss on debt extinguishment | — | | | | 15.9 | | | — | | | — | | | 12.8 | |
| Foreign currency loss (gain), net | 1.6 | | | | (0.6) | | | 13.4 | | | (0.6) | | | (3.2) | |
| Change in fair value of warrant liabilities | (1.2) | | | | — | | | — | | | — | | | — | |
| Other expense (income), net | 0.3 | | | | 1.6 | | | (1.1) | | | (1.0) | | | 1.9 | |
| Loss before benefit from income taxes | (29.8) | | | | (111.3) | | | (164.3) | | | (124.6) | | | (126.2) | |
| Benefit from income taxes | (6.8) | | | | (5.6) | | | (5.9) | | | (5.5) | | | (4.2) | |
| Net loss | (23.0) | | | | (105.7) | | | (158.4) | | | (119.1) | | | (122.0) | |
| Loss attributable to noncontrolling interests | (0.8) | | | | — | | | (0.1) | | | — | | | — | |
| Net loss attributable to Mirion Technologies, Inc. (Successor) / Mirion Technologies (TopCo), Ltd. (Predecessor) stockholders | $ | (22.2) | | | | $ | (105.7) | | | $ | (158.3) | | | $ | (119.1) | | | $ | (122.0) | |
| Net loss per common share attributable to Mirion Technologies, Inc. (Successor) / Mirion Technologies (TopCo), Ltd. (Predecessor) stockholders — basic and diluted | $ | (0.12) | | | | $ | (15.81) | | | $ | (24.18) | | | $ | (18.45) | | | $ | (19.36) | |
| Weighted average common shares outstanding — basic and diluted | 180.773 | | | 6.685 | | 6.549 | | 6.453 | | 6.300 |
The accompanying notes are an integral part of these consolidated financial statements.
Mirion Technologies, Inc.
Consolidated Statements of Comprehensive Loss
(In millions)
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Successor | | | Predecessor |
| From
October 20, 2021
through
December 31, 2021 | | | From
July 1, 2021
through
October 19, 2021 | | Fiscal Year Ended
06/30/2021 | | Fiscal Year Ended
06/30/2020 | | Fiscal Year Ended
06/30/2019 |
| Net loss | $ | (23.0) | | | | $ | (105.7) | | | $ | (158.4) | | | $ | (119.1) | | | $ | (122.0) | |
| Other comprehensive loss, net of tax: | | | | | | | | | | |
| Foreign currency translation, net of tax | (20.5) | | | | (7.5) | | | 34.2 | | | (9.3) | | | (15.1) | |
| Unrecognized actuarial (loss) gain and prior service benefit, net of tax | (0.2) | | | | 0.6 | | | 0.9 | | | — | | | (1.5) | |
| Other comprehensive (loss) income, net of tax | (20.7) | | | | (6.9) | | | 35.1 | | | (9.3) | | | (16.6) | |
| Comprehensive loss | (43.7) | | | | (112.6) | | | (123.3) | | | (128.4) | | | (138.6) | |
| Less: Comprehensive loss attributable to noncontrolling interest | (0.8) | | | | — | | | (0.1) | | | — | | | — | |
| Comprehensive loss attributable to Mirion Technologies, Inc. (Successor) / Mirion Technologies (TopCo), Ltd. (Predecessor) stockholders | $ | (42.9) | | | | $ | (112.6) | | | $ | (123.2) | | | $ | (128.4) | | | $ | (138.6) | |
The accompanying notes are an integral part of these consolidated financial statements.
Mirion Technologies, Inc.
Consolidated Statements of Stockholders’ Equity (Deficit)
(In millions, except share amounts)
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Predecessor | A Ordinary
Shares | | A Ordinary
Amount | | B Ordinary
Shares | | B Ordinary
Amount | | Additional
Paid-In
Capital | | Receivable from
Employees for
purchase of
Common Stock | | Accumulated
Deficit | | Accumulated Other
Comprehensive
Income (Loss) | | Noncontrolling
Interests | | Total
Stockholders’
Deficit |
| Balance July 1, 2018 | 1,483,795 | | | $ | — | | | 5,353,970 | | | $ | 0.1 | | | $ | 7.3 | | | $ | (0.2) | | | $ | (488.6) | | | $ | 30.0 | | | $ | 2.6 | | | $ | (448.8) | |
| Contribution from noncontrolling interests | — | | | — | | | — | | | — | | | — | | | — | | | — | | | — | | | 0.1 | | | 0.1 | |
| Distribution to noncontrolling interests | — | | | — | | | — | | | — | | | — | | | — | | | — | | | — | | | (0.1) | | | (0.1) | |
| Share-based compensation expense | — | | | — | | | — | | | — | | | 0.1 | | | — | | | — | | | — | | | — | | | 0.1 | |
| Receivable from Employees | — | | | — | | | — | | | — | | | 1.9 | | | (2.3) | | | — | | | — | | | — | | | (0.4) | |
| Net loss | — | | | — | | | — | | | — | | | — | | | — | | | (122.0) | | | — | | | — | | | (122.0) | |
| Other comprehensive loss | — | | | — | | | — | | | — | | | — | | | — | | | — | | | (16.6) | | | — | | | (16.6) | |
| Balance June 30, 2019 | 1,483,795 | | | — | | | 5,353,970 | | | 0.1 | | | 9.3 | | | (2.5) | | | (610.6) | | | 13.4 | | | 2.6 | | | (587.7) | |
| Distribution to noncontrolling interests | — | | | — | | | — | | | — | | | — | | | — | | | — | | | — | | | (0.4) | | | (0.4) | |
| Share-based compensation expense | — | | | — | | | — | | | — | | | 0.2 | | | — | | | — | | | — | | | — | | | 0.2 | |
| Receivable from Employees | — | | | — | | | — | | | — | | | — | | | (0.2) | | | — | | | — | | | — | | | (0.2) | |
| Net loss | — | | | — | | | — | | | — | | | — | | | — | | | (119.1) | | | — | | | — | | | (119.1) | |
| Other comprehensive loss | — | | | — | | | — | | | — | | | — | | | — | | | — | | | (9.3) | | | — | | | (9.3) | |
| Balance June 30, 2020 | 1,483,795 | | | — | | | 5,353,970 | | | 0.1 | | | 9.5 | | | (2.7) | | | (729.7) | | | 4.1 | | | 2.2 | | | (716.5) | |
| Receivable from Employees | — | | | — | | | — | | | — | | | — | | | 0.3 | | | — | | | — | | | — | | | 0.3 | |
| Net loss | — | | | — | | | — | | | — | | | — | | | — | | | (158.3) | | | — | | | (0.1) | | | (158.4) | |
| Other comprehensive income | — | | | — | | | — | | | — | | | — | | | — | | | — | | | 35.1 | | | — | | | 35.1 | |
| Balance June 30, 2021 | 1,483,795 | | | $ | — | | | 5,353,970 | | | $ | 0.1 | | | $ | 9.5 | | | $ | (2.4) | | | $ | (888.0) | | | $ | 39.2 | | | $ | 2.1 | | | $ | (839.5) | |
| Share-based compensation expense | — | | | — | | | — | | | — | | | 9.3 | | | — | | | — | | | — | | | — | | | 9.3 | |
| Impairment loss on lease adoption | — | | | — | | | — | | | — | | | — | | | — | | | (2.9) | | | — | | | — | | | (2.9) | |
| Receivable from employees | — | | | — | | | — | | | — | | | — | | | 1.6 | | | — | | | — | | | — | | | 1.6 | |
| Net loss | — | | | — | | | — | | | — | | | — | | | — | | | (105.7) | | | — | | | — | | | (105.7) | |
| Other comprehensive loss | — | | | — | | | — | | | — | | | — | | | — | | | — | | | (6.9) | | | — | | | (6.9) | |
| Balance October 19, 2021 | 1,483,795 | | | $ | — | | | 5,353,970 | | | $ | 0.1 | | | $ | 18.8 | | | $ | (0.8) | | | $ | (996.6) | | | $ | 32.3 | | | $ | 2.1 | | | $ | (944.1) | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Successor | Class A Common Stock | | Class A Common Stock Amount | | Class B Common Stock | | Class B Common Stock Amount | | Additional Paid-In Capital | | Accumulated Deficit | | Accumulated Other Comprehensive Income (Loss) | | Noncontrolling Interests | | Total Stockholders’ Deficit |
| Balance October 20, 2021 | — | | | $ | — | | | 18,750,000 | | | $ | — | | | $ | — | | | $ | (109.4) | | | $ | — | | | $ | — | | | $ | (109.4) | |
| Conversion of Class B Founder Shares to Class A Common Shares upon Business Combination | 18,750,000 | | | — | | | (18,750,000) | | | — | | | — | | | — | | | — | | | — | | | — | |
| Reclassification of temporary equity shares previously subject to redemption | 60,371,390 | | | — | | | — | | | — | | | 603.7 | | | — | | | — | | | — | | | 603.7 | |
| Issuance of Class A Common Shares to PIPE Investors, net of offering costs | 90,000,000 | | | — | | | — | | | — | | | 886.7 | | | — | | | — | | | — | | | 886.7 | |
| Issuance of Common Shares to Mirion Sellers and recognition of noncontrolling interests in Mirion Business Combination | 30,401,902 | | | — | | | 8,560,540 | | | — | | | 329.1 | | | — | | | — | | | 91.6 | | | 420.7 | |
| Equity contribution from Mirion Sellers | — | | | — | | | — | | | — | | | 18.7 | | | — | | | — | | | — | | | 18.7 | |
| Forgiveness of working capital note from Sponsor | — | | | — | | | — | | | — | | | 2.0 | | | — | | | — | | | — | | | 2.0 | |
| Stock-based compensation expense | — | | | — | | | — | | | — | | | 5.3 | | | — | | | — | | | — | | | 5.3 | |
| Net loss | — | | | — | | | — | | | — | | | — | | | (22.2) | | | — | | | (0.8) | | | (23.0) | |
| Other comprehensive loss | — | | | — | | | — | | | — | | | — | | | — | | | (20.7) | | | — | | | (20.7) | |
| Balance December 31, 2021 | 199,523,292 | | | $ | — | | | 8,560,540 | | | $ | — | | | $ | 1,845.5 | | | $ | (131.6) | | | $ | (20.7) | | | $ | 90.8 | | | $ | 1,784.0 | |
The accompanying notes are an integral part of these consolidated financial statements.
Mirion Technologies, Inc.
Consolidated Statements of Cash Flows
(In millions)
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Successor | | | Predecessor |
| | Transition
Period from
October 20, 2021
through
December 31, 2021 | | | From July 1, 2021 through
October 19, 2021 | | Fiscal Year Ended
June 30, 2021 | | Fiscal Year Ended
June 30, 2020 | | Fiscal Year Ended
June 30, 2019 |
| OPERATING ACTIVITIES: | | | | | | | | | | |
| Net loss | $ | (23.0) | | | | $ | (105.7) | | | $ | (158.4) | | | $ | (119.1) | | | $ | (122.0) | |
| Adjustments to reconcile net loss to net cash provided by operating activities: | | | | | | | | | | |
| Accrual of in-kind interest on notes payable to related parties | — | | | | 40.2 | | | 121.2 | | | 107.7 | | | 95.6 | |
| Depreciation and amortization expense | 37.3 | | | | 25.9 | | | 83.6 | | | 68.4 | | | 69.5 | |
| Stock-based compensation expense | 5.3 | | | | 9.3 | | | — | | | 0.2 | | | 0.1 | |
| Loss on debt extinguishment | — | | | | 15.9 | | | — | | | — | | | 12.8 | |
| Amortization of debt issuance costs | 0.7 | | | | 1.1 | | | 3.2 | | | 2.6 | | | 3.6 | |
| Provision for doubtful accounts | (0.8) | | | | 0.3 | | | 2.1 | | | 0.6 | | | 0.5 | |
| Inventory obsolescence write down | 0.3 | | | | — | | | 0.7 | | | 1.9 | | | — | |
| Change in deferred income taxes | (11.2) | | | | (8.4) | | | (16.6) | | | (15.5) | | | (16.1) | |
| Loss (gain) on disposal of property, plant and equipment | 0.8 | | | | 1.6 | | | (0.1) | | | 0.4 | | | 1.2 | |
| Loss (gain) on foreign currency transactions | 1.6 | | | | (0.6) | | | 13.4 | | | (1.7) | | | 2.7 | |
| Change in fair values of warrant liabilities | (1.2) | | | | — | | | — | | | — | | | — | |
| Amortization of deferred revenue step-down | 2.3 | | | | 4.5 | | | 8.0 | | | 0.2 | | | — | |
| Amortization of inventory step-up | 15.8 | | | | — | | | 5.2 | | | 1.6 | | | 0.1 | |
| Other | (0.1) | | | | — | | | 1.4 | | | (0.9) | | | (0.1) | |
| Changes in operating assets and liabilities: | | | | | | | | | | |
| Accounts receivable | (42.5) | | | | 18.2 | | | (4.2) | | | 3.8 | | | 10.6 | |
| Costs in excess of billings on uncompleted contracts | 6.3 | | | | (5.7) | | | (3.8) | | | (2.9) | | | (8.1) | |
| Inventories | 5.1 | | | | (10.2) | | | (4.2) | | | 2.7 | | | (7.9) | |
| Deferred cost of revenue | (0.3) | | | | (0.4) | | | 6.6 | | | (3.5) | | | (0.2) | |
| Prepaid expenses and other current assets | (2.5) | | | | 2.6 | | | (10.1) | | | (1.6) | | | 1.4 | |
| Accounts payable | (8.9) | | | | 19.2 | | | 2.6 | | | (2.5) | | | (2.7) | |
| Accrued expenses and other current liabilities | (8.4) | | | | 0.4 | | | (2.2) | | | 7.3 | | | (13.1) | |
| Deferred contract revenue | 10.6 | | | | 4.5 | | | (2.8) | | | (1.9) | | | (8.4) | |
| Other assets | (6.1) | | | | (2.2) | | | 0.5 | | | 0.2 | | | 0.5 | |
| Other liabilities | 6.7 | | | | 2.6 | | | 7.5 | | | (8.5) | | | (5.3) | |
| Net cash provided by operating activities | (12.2) | | | | 13.1 | | | 53.6 | | | 39.5 | | | 14.7 | |
| INVESTING ACTIVITIES: | | | | | | | | | | |
| Acquisition of Mirion Topco, net of cash and cash equivalents acquired | $ | (2,124.8) | | | | — | | | — | | | — | | | — | |
| Acquisitions of businesses, net of cash and cash equivalents acquired | (58.6) | | | | (0.9) | | | (290.1) | | | (55.7) | | | (9.1) | |
| Purchases of property, plant, and equipment and badges | (6.0) | | | | (11.6) | | | (23.2) | | | (19.9) | | | (16.5) | |
| Net cash used in investing activities | (2,189.4) | | | | (12.5) | | | (313.3) | | | (75.6) | | | (25.6) | |
| FINANCING ACTIVITIES: | | | | | | | | | | |
| Issuances of common stock | $ | 900.0 | | | | — | | | — | | | — | | | — | |
| Common stock issuance costs | (13.3) | | | | — | | | — | | | — | | | — | |
| Transaction fees reimbursed by Sellers | 18.7 | | | | — | | | — | | | — | | | — | |
| Payment of deferred underwriting costs | (26.3) | | | | — | | | — | | | — | | | — | |
| SPAC share redemption | (146.3) | | | | — | | | — | | | — | | | — | |
| Borrowings from notes payable to third-parties, net of discount and issuance costs | 807.3 | | | | 1.9 | | | 218.8 | | | 98.8 | | | 596.8 | |
| Principal repayments | (1.7) | | | | (2.4) | | | (14.8) | | | (13.4) | | | (560.2) | |
| Deferred finance costs | (0.9) | | | | — | | | — | | | — | | | (8.1) | |
| Borrowings from notes payable – related parties | — | | | | — | | | 70.0 | | | — | | | — | |
| Borrowing on revolving term loan | — | | | | — | | | — | | | 80.0 | | | — | |
| Payment on revolving term loan | — | | | | — | | | (35.0) | | | (45.0) | | | (13.0) | |
| Payment of contingent considerations | — | | | | — | | | — | | | (2.0) | | | — | |
| Contribution from noncontrolling interests | — | | | | — | | | — | | | — | | | 0.1 | |
| Distributions to noncontrolling interests | — | | | | — | | | — | | | (0.4) | | | (0.1) | |
| Other financing | 0.2 | | | | 1.5 | | | — | | | 0.9 | | | (0.5) | |
| Net cash provided by financing activities | 1,537.7 | | | | 1.0 | | | 239.0 | | | 118.9 | | | 15.0 | |
| Effect of exchange rate changes on cash, cash equivalents, and restricted cash | (1.0) | | | | (0.9) | | | 3.1 | | | (0.4) | | | (2.4) | |
| Net (decrease) increase in cash, cash equivalents, and restricted cash | (664.9) | | | | 0.7 | | | (17.6) | | | 82.4 | | | 1.7 | |
| Cash, cash equivalents, and restricted cash at beginning of period | 750.2 | | | | 102.4 | | | 120.0 | | | 37.6 | | | 35.9 | |
| Cash, cash equivalents, and restricted cash at end of period | $ | 85.3 | | | | $ | 103.1 | | | $ | 102.4 | | | $ | 120.0 | | | $ | 37.6 | |
The accompanying notes are an integral part of these consolidated financial statements.
Mirion Technologies, Inc.
Notes to Consolidated Financial Statements
1. Nature of Business and Summary of Significant Accounting Policies
Nature of Business
Mirion Technologies, Inc. (“Mirion”, the “Company” or "Successor" or "us" and formerly GS Acquisition Holdings Corp II ("GSAH")) is a global provider of radiation detection, measurement, analysis, and monitoring products and services to the medical, nuclear, and defense end markets. We provide products and services through our two operating and reportable segments; (i) Medical and (ii) Industrial. The medical segment provides radiation oncology quality assurance, delivering patient safety solutions for diagnostic imaging and radiation therapy centers around the world, dosimetry solutions for monitoring the total amount of radiation medical staff members are exposed to over time, radiation therapy quality assurance solutions for calibrating and verifying imaging and treatment accuracy, and radionuclide therapy products for nuclear medicine applications such as shielding, product handling, medical imaging furniture, and rehabilitation products. The industrial segment provides robust, field ready personal radiation detection and identification equipment for defense applications and radiation detection and analysis tools for power plants, labs, and research applications. Nuclear power plant product offerings are used for the full nuclear power plant lifecycle including core detectors and essential measurement devices for new build, maintenance, decontamination and decommission equipment for monitoring and control during fuel dismantling and remote environmental monitoring.
The Company is headquartered in Atlanta, Georgia and has operations in the United States, Canada, the United Kingdom, France, Germany, Finland, China, Belgium, Netherlands, Estonia, and Japan.
On October 20, 2021 (the “Closing Date”), the Company, consummated its previously announced business combination (the “Business Combination”) pursuant to the certain business combination agreement (the "Business Combination Agreement"). In connection with the Business Combination, stockholders of GSAH elected to redeem 14,628,610 shares of Class A common stock, par value $0.0001 per share, of the Company (the “Class A common stock”), representing approximately 19.5% of the Company’s issued and outstanding Class A common stock before giving effect to the Business Combination.
GSAH was originally incorporated as a Delaware corporation on May 31, 2018 for the purpose of effecting a merger, capital stock exchange, asset acquisition, stock purchase, reorganization or similar business combination with one or more businesses. GSAH units, each of which consisted of one share of Class A common stock and one fourth of one warrant were sold in GSAH's initial public offering on June 29, 2020. GSAH units, Class A common stock and warrants were listed on the New York Stock Exchange (the "NYSE") under the symbols, "GSAH.U", "GSAH" and "GSAH.WS", respectively. On the Closing Date, GSAH was renamed Mirion Technologies, Inc. Our Class A common stock and warrants are listed on the NYSE under the ticker symbols “MIR” and "MIR WS", respectively.
As contemplated by the Business Combination Agreement, the Company became the corporate parent of Mirion Technologies TopCo., Ltd. ("Mirion TopCo"). In order to implement a structure similar to that of an “Up-C,” the Company established a Delaware corporation, Mirion IntermediateCo, Inc. (“IntermediateCo”), as a subsidiary of the Company.
The aggregate business combination consideration (the “Business Combination Consideration”) paid by the Company to the selling shareholders of Mirion TopCo (the "Sellers") in connection with the consummation of the Business Combination was $1.3 billion in cash, 30,401,902 newly issued shares of Class A common stock and 8,560,540 newly issued shares of the Company’s Class B common stock that have voting rights but no economic interest in the Company, par value $0.0001 per share (the “Class B common stock” and, together with the Class A common stock, the “Common Stock”).
Basis of Presentation and Principles of Consolidation
The accompanying Consolidated Financial Statements and Notes to Consolidated Financial Statements have been prepared in accordance with accounting principles generally accepted in the United States of America (“GAAP”) for financial statements and pursuant to the accounting and disclosure rules and regulations of the U.S. Securities and Exchange Commission (the "SEC"). The Consolidated Financial Statements include the accounts of the Company and its wholly owned and majority-owned or controlled subsidiaries. For consolidated subsidiaries where our ownership is less than 100%, the portion of the net income or loss allocable to noncontrolling interests is reported as “Income (Loss) attributable
to noncontrolling interests” in the Consolidated Statements of Operations. All intercompany accounts and transactions have been eliminated in consolidation.
The Company recognizes a noncontrolling interest for the portion Class B common stock of IntermediateCo that is not attributable to the Company. See Note 20, Noncontrolling Interests.
On October 20, 2021, the Board of Directors determined to change Mirion TopCo's fiscal year end from June 30 of each year to December 31 of each year in order to align Mirion’s fiscal year end with GSAH’s fiscal year end.
Predecessor and Successor Reporting
The financial statements separate the Company’s presentation into two distinct periods. The period before the Closing Date of the Business Combination (the "Predecessor Period") depicts the financial statements of Mirion TopCo, and the period after the Closing (the "Successor Period") depicts the financial statements of the Company, including the consolidation of GSAH with Mirion Technologies, Inc.
The Business Combination is being accounted for under ASC 805, Business Combinations. GSAH has been determined to be the accounting acquirer. Mirion Technologies, Inc. constitutes a business in accordance with ASC 805 and the business combination constitutes a change in control. Accordingly, the Business Combination is being accounted for using the acquisition method. Under this method of accounting, Mirion TopCo is treated as the “acquired” company for financial reporting purposes and our net assets are stated at fair value, with goodwill or other intangible assets recorded. Refer to Note 2, Acquisitions, for further detail.
As a result of the application of the acquisition method of accounting in the Successor Period, the financial statements for the Successor Period are presented on a full step-up basis as a result of the Business Combination, and are therefore not comparable to the financial statements of the Predecessor Period.
Filing Status
Mirion qualified as a large accelerated filer following the end of its fiscal year ended December 31, 2021. Before such time, the Company qualified as an emerging growth company. Section 102(b)(1) of the JOBS Act exempts emerging growth companies from being required to comply with new or revised financial accounting standards until private companies (that is, those that have not had a Securities Act registration statement declared effective or do not have a class of securities registered under the Exchange Act) are required to comply with the new or revised financial accounting standards. The JOBS Act provides that a company can elect to opt out of the extended transition period and comply with the requirements that apply to non-emerging growth companies but any such an election to opt out is irrevocable. The Company historically elected not to opt out of such extended transition period which means that when a standard is issued or revised and it has different application dates for public or private companies, the Company, as an emerging growth company, adopted the new or revised standard at the time private companies adopted the new or revised standard.
This may make comparison of the Company’s financial statements for historical periods with those of another public company that is neither an emerging growth company nor an emerging growth company that has opted out of using the extended transition period difficult or impossible because of the potential differences in accounting standards used.
Segments
The Company manages its operations through two operating and reportable segments: Medical and Industrial. These segments align the Company’s products and service offerings with customer use in medical and industrial markets and are consistent with how the Company’s Chief Executive Officer, its Chief Operating Decision Maker (“CODM”), reviews and evaluates the Company’s operations. The CODM allocates resources and evaluates the financial performance of each operating segment. The Company’s segments are strategic businesses that are managed separately because each one develops, manufactures and markets distinct products and services. Refer to Note 16, Segments, for further detail.
Use of Estimates
Management estimates and judgments are an integral part of financial statements prepared in accordance with GAAP. We believe that the critical accounting policies listed below address the more significant estimates required of management when preparing our consolidated financial statements in accordance with GAAP. We consider an accounting estimate critical if changes in the estimate may have a material impact on our financial condition or results of operations. We believe that the accounting estimates employed are appropriate and resulting balances are reasonable; however, actual results could differ from the original estimates, requiring adjustment to these balances in future periods. The accounting
policies that reflect our more significant estimates, judgments and assumptions and which we believe are the most critical to aid in fully understanding and evaluating our reported financial results include but are not limited to: business combinations, goodwill and intangible assets; standalone selling prices for revenue arrangements with multiple elements and estimated progress toward completion for certain revenue contracts; uncertain tax positions and tax valuation allowances and derivative warrant liabilities.
Cash and Cash Equivalents
The Company considers all cash on deposit and money market accounts purchased with original maturities of three months or less to be cash and cash equivalents. Cash equivalents primarily consist of amounts held in interest-bearing money market accounts that are readily convertible to cash.
The Company maintains cash in bank deposit accounts that, at times, may exceed the insured limits of the local country, which may lead to a concentration of credit risk. Substantially all of the Company’s cash and cash equivalent balances were deposited with financial institutions which management has determined to be high-credit quality institutions. The Company has not experienced any losses in such accounts.
Restricted Cash
The Company maintains restricted cash and cash equivalent accounts with various financial institutions to support performance bonds with irrevocable letters of credit for contractual obligations to certain customers. As of December 31, 2021, June 30, 2021, and June 30, 2020 combined current and non-current restricted cash on the consolidated balance sheets was $1.3 million, $1.3 million, and $1.6 million respectively.
Accounts Receivable and Allowance for Doubtful Accounts
Accounts receivable are recorded at the invoiced amount and do not bear interest. The allowance for doubtful accounts is based on the Company’s assessment of the collectability of customer accounts. The Company regularly reviews the allowance by considering factors such as historical experience, credit quality, the age of the accounts receivable balances and current economic conditions that may affect a customer’s ability to pay. The allowance for doubtful accounts was $5.4 million, $6.1 million, and $1.9 million as of December 31, 2021, June 30, 2021, and June 30, 2020 respectively.
Inventories
Inventories are stated at the lower of cost or net realizable value. Cost is computed using actual costs or standard costs that approximate actual cost, determined on a first-in, first-out basis. A portion of the inventory relates to evaluation units located at customer locations to facilitate customer tests prior to purchasing. Inventories also include completed products and in-process customer projects for which the related revenue has been deferred pending delivery, completion of services or determination that all customer-specific acceptance criteria have been met. Inventory in excess of expected future demand or obsolete inventory is written down to its estimated realizable value based on future demand forecasts and historical demand trends.
Deferred Cost of Revenue
Deferred cost of revenue consists of the direct costs associated with production for identified projects for which the revenue has been deferred in accordance with the Company’s revenue recognition policies. Deferred costs are recognized as cost of revenues in the same period that the related revenues are recognized.
Other Current Assets
Other current assets are primarily comprised of various prepaid assets including prepaid insurance, short-term marketable securities, and income tax receivables. The prepaid insurance was $5.3 million, $0.8 million, and $0.3 million as of December 31, 2021, June 30, 2021, and June 30, 2020, respectively. The short-term marketable securities were $4.9 million, $4.6 million, and $3.5 million as of December 31, 2021, June 30, 2021, and June 30, 2020, respectively. The income tax receivables were $2.8 million, $3.6 million, and $0.5 million as of December 31, 2021, June 30, 2021, and June 30, 2020, respectively.
Lease Assets
We adopted the provisions of the Financial Accounting Standards Board ("FASB") Accounting Standards Codification ("ASC") 842 on July 1, 2021 using the modified retrospective approach and, as a result, did not restate prior periods. The Company leases certain logistics, office, and manufacturing facilities, as well as vehicles, copiers and other equipment. We record our operating lease right of use ("ROU") assets and liabilities at the commencement date of the lease based on the present value of lease payments over the lease term.
ROU assets represent our right to use an underlying asset for the lease term and lease liabilities represent our obligation to make lease payments arising from the lease. Our leases may include options to extend or terminate the lease. These options to extend are included in the lease term when it is reasonably certain that we will exercise that option. While some leases provide for variable payments, they are not included in the ROU assets and liabilities because they are not based on an index or rate. Variable payments for real estate leases primarily relate to common area maintenance, insurance, taxes and utilities. Variable payments for equipment, vehicles and leases within supply agreements primarily relate to usage, repairs, and maintenance. As the implicit rate is not readily determinable for our leases, we apply a portfolio approach using an estimated incremental borrowing rate to determine the initial present value of lease payments over the lease terms on a collateralized basis over a similar term, which is based on market and company specific information. We use the unsecured borrowing rate and risk-adjust that rate to approximate a collateralized rate, and apply the rate based on the currency of the lease, which is updated on a quarterly basis for measurement of new lease liabilities.
We have made an accounting policy election to not recognize ROU assets and liability for leases with a term of 12 months or less unless the lease includes an option to renew or purchase the underlying asset that are reasonably certain to be exercised. In addition, the Company has applied the practical expedient to account for the lease and non-lease components as a single lease component for all of the Company's leases.
See Note 9, Leased Assets for additional details.
Property, Plant, and Equipment
Property, plant, and equipment are carried at cost, net of accumulated depreciation and amortization. Property, plant and equipment acquired through the acquisition of a business are recorded at their estimated fair value at the date of acquisition.
Depreciation is computed when an asset is placed into service using the straight-line method over the estimated useful life of the asset. The Company capitalizes costs incurred in the acquisition and development of software for internal use, including the costs of software, materials, consultants, and payroll-related costs of employees incurred in developing internal-use computer software. Development costs related to internal-use software are amortized using the straight-line method over the shorter of the software license or the estimated useful life of the software. Leasehold improvements are amortized using the straight-line method over the shorter of the related lease term or the estimated useful life of the improvements. Repair and maintenance costs are expensed as incurred.
Estimated useful lives are periodically reviewed and, when appropriate, changes to estimates are made prospectively. When certain events or changes in operating conditions occur, asset lives may be adjusted, and an impairment assessment may be performed on the recoverability of the carrying amounts. Refer to Note 5, Property, Plant and Equipment, net, for disclosure of estimated useful lives.
When property, plant equipment is retired or otherwise disposed of, the cost and related accumulated depreciation are removed from the balance sheet. Any difference between the net asset value and the proceeds on sale are charged or credited to income.
Business Combinations
We account for business acquisitions in accordance with ASC 805, "Business Combinations". This standard requires the acquiring entity in a business combination to recognize all the assets acquired and liabilities assumed in the transaction and establishes the acquisition date fair value as the measurement objective for all assets acquired and liabilities assumed in a business combination. Certain provisions of this standard prescribe, among other things, the determination of acquisition date fair value of consideration paid in a business combination (including contingent consideration) and the exclusion of transaction and acquisition-related restructuring costs from acquisition accounting.
The determination of the fair value of assets acquired and liabilities assumed involves assessments of factors such as the expected future cash flows associated with individual assets and liabilities and appropriate discount rates at the closing date
of the acquisition. For non-observable market values, the Company determines fair value using acceptable valuation principles (e.g., multiple excess earnings, relief from royalty and cost methods).
Results of operations for acquired companies are included in our consolidated results of operations from the date of acquisition.
Goodwill
Goodwill represents the excess of the purchase price paid over the estimated fair value of the net assets acquired and liabilities assumed in the acquisition of a business.
Goodwill has an indefinite useful life, and is not amortized, but instead tested for impairment annually during the fiscal year fourth quarter or more often if events or changes in circumstances indicate that the carrying amount may exceed fair value as set forth in ASC 350, “Intangibles — Goodwill and Other.” The Company tests for goodwill impairment at the reporting unit level, which is an operating segment or one level below an operating segment. The amount of goodwill acquired in a business combination that is assigned to one or more reporting units as of the acquisition date is the excess of the purchase price of the acquired businesses (or portion thereof) included in the reporting unit, over the fair value assigned to the individual assets acquired or liabilities assumed from a market participant perspective. Goodwill is assigned to the reporting unit(s) expected to benefit from the synergies of the combination even though other assets or liabilities of the acquired entity may not be assigned to that reporting unit.
ASC 350 allows an optional qualitative assessment as part of annual impairment testing, prior to a quantitative assessment test, to determine whether it is more likely than not that the fair value of a reporting unit exceeds its carrying amount. If a qualitative assessment determines an impairment is more likely than not, the Company is required to perform a quantitative impairment test. Otherwise, no further analysis is required. Alternatively, the Company may elect to proceed directly to the quantitative impairment test.
In conducting a qualitative assessment, the Company analyzes actual and projected growth trends for net sales and margin for each reporting unit, as well as historical performance versus plan and the results of prior quantitative tests performed. Additionally, the Company assesses factors that may impact its business, including macroeconomic conditions and the related impact, market-related exposures, plans to market for sale all or a portion of the business, competitive changes, new or discontinued product lines, changes in key personnel, and any potential risks to projected financial results.
If performed, the quantitative test compares the fair value of a reporting unit with its carrying amount. We determine the fair value of each reporting unit by estimating the present value of expected future cash flows, discounted by the applicable discount rate, and peer company multiples. If the carrying value exceeds the fair value, the Company recognizes an impairment loss in the amount equal to the excess, not to exceed the total amount of goodwill allocated to that reporting unit.
Based upon our review and analysis, no impairments were deemed to have occurred during any of the years presented. Refer to Note 7, Goodwill and Intangible Assets, for further detail.
Intangible Assets
Intangible assets relate to the value associated with our developed technology, customer relationships, backlog, and trade names at the time of acquisition through business combinations.
The Company determined the fair value of intangible assets acquired through an income approach, using the excess earnings method for customer relationships and backlog. Under the excess earnings method, an intangible asset’s fair value is equal to the present value of the incremental after-tax cash flows attributable solely to the intangible asset over its remaining useful life. The relief from royalty method was used to determine the fair value of developed technology and tradename. The valuation models were based on estimates of future operating projections of the acquired business and rights to sell products as well as judgments on the discount rates used and other variables. We determined the forecasts based on a number of factors, including our best estimate of near-term net sales expectations and long-term projections, which include review of internal and independent market analyses. The discount rate used was representative of the weighted average cost of capital.
The customer relationships definite lived intangible assets are amortized using the double declining balance method with estimated useful lives ranging from 6 to 13 years, while all other definite lived intangible assets are amortized on a straight-line basis over their estimated useful lives, ranging from 5 to 16 years for developed technology and 1 to 10 years for
tradenames and other. The Company regularly evaluates the amortization period assigned to each intangible asset to ensure that there have not been any events or circumstances that warrant revised estimates of useful lives. Refer to Note 7, Goodwill and Intangible Assets, for further detail.
Impairment of Long-Lived Assets
The Company reviews long-lived assets and definite-lived intangible assets for impairment whenever events or changes in circumstances indicate that the carrying amount of an asset may not be recoverable. If an evaluation of recoverability is required, the estimated undiscounted future cash flows associated with the asset group are compared to the asset group’s carrying amount to determine if a write-down is required. If the undiscounted cash flows are less than the carrying amount, an impairment loss is recorded to the extent that the carrying amount exceeds the fair value. No impairment was recorded during any periods or fiscal years presented.
Facility and Equipment Decommissioning Liabilities
The Company has asset retirement obligations (“ARO”) consisting primarily of equipment and facility decommissioning costs. The estimated fair value of these ARO liabilities is recognized in the period in which the liability is generated and a corresponding increase to the carrying value of the related asset is recorded and depreciated over the useful life of the asset. The Company’s estimates of its ultimate AROs could change because of changes in regulations, the extent of environmental remediation required, the means of reclamation, cost estimates, exit or disposal activities or time period estimates.
ARO liabilities totaled $3.1 million, $3.7 million, and $4.0 million at December 31, 2021, June 30, 2021, and June 30, 2020, respectively, and were included in deferred income taxes and other liabilities on the consolidated balance sheets. Accretion expense related to these liabilities was not material for any periods or fiscal years presented .
Product Warranty
The Company offers warranties against material defects for most of its products for a specified time period, usually twelve to twenty-four months from delivery or acceptance. When the related revenues are recognized, the Company provides for the estimated future costs of warranty obligations in cost of revenues. The accrued warranty costs represent the Company’s best estimate at the time of sale of the total costs that will be incurred to repair or replace product parts that fail while still under warranty.
The amount of the accrued estimated warranty cost obligations for established products is based on historical experience as to product failures adjusted for current information on repair costs. For new products, estimates include the historical experience of similar products, as well as a reasonable allowance for warranty expenses associated with the new products. On a quarterly basis, the Company reviews the accrued warranty costs and updates the historical warranty cost trends, if required.
Revenue Recognition
Prior to July 1, 2019, the Company recognized revenue based on ASC 605, when there was persuasive evidence of an arrangement, product delivery had occurred or services had been provided, the sales price was fixed or determinable and collectability was reasonably assured. Beginning on July 1, 2019, the Company recognizes revenue based on ASC 606 as performance obligations are satisfied by transferring control of promised goods or service to customers in an amount that reflects the consideration to which the company expects to be entitled in exchange for those goods or services. See "Recently Adopted Accounting Guidance" below for a discussion of the change in revenue recognition accounting that became effective on July 1, 2019.
ASC 606
The Company recognizes revenue from arrangements that include performance obligations to design, engineer, manufacture, deliver, and install products. The Company identifies a performance obligation for each promise in a contract to transfer a distinct good or service to the customer. As part of its assessment, the Company considers all goods and/or services promised in the contract, regardless of whether they are explicitly stated or implied by customary business practices. The Company’s contracts may contain either a single performance obligation, including the promise to transfer individual goods or services that are not separately distinct within the context of the respective contracts, or multiple performance obligations. For contracts that contain multiple performance obligations, the Company allocates the consideration to which it expects to be entitled to each performance obligation based on relative standalone selling prices
and recognizes the related revenue when or as control of each individual performance obligation is transferred to customers. The Company does not assess whether promised goods or services are performance obligations if they are immaterial in the context of the contract with the customer. The Company combines multiple contracts entered into at or around the same time with a customer if the contracts are negotiated as a package with a single commercial objective, the consideration paid under the contracts depends on the price or performance of the other contract, or if the goods or services promised in the contracts are a single performance obligation. Service revenues (service-type warranty, post contract support, installation, and subscription-based services) are recognized over time as the customers receive and consume benefits of such services simultaneously. Assurance-type warranties guarantee that a product complies with agreed-upon specifications and accordingly are not separate performance obligations. A provision for these warranties is recognized in the period during which the associated revenue is recognized. In most cases, installation services represent a separate performance obligation. The customer simultaneously receives and consumes the benefits as the installation services are performed, as other entities could complete the installation at any point during the installation process. When the product and installation service are determined to be a combined performance obligation, revenue is recognized over time as the installation is performed and included in product revenue in the consolidated statement of operations.
Variable consideration such as rebates, sales discounts and sales returns are estimated and treated as a reduction of revenue in the same period the related revenue is recognized. These are estimated based on contractual terms, historical practices, and current trends, and are adjusted as new information becomes available. Revenues exclude any taxes that the Company collects from customers and remits to tax authorities. Amounts billed to customer for shipping and handling are included in revenue, while the related shipping and handling costs are reflected in cost of products in the period in which revenue is recognized. The Company has elected a practical expedient under ASC 606 that allows for shipping and handling activities that occur after the customer has obtained control of a good to be accounted for as a fulfillment cost. The Company does not adjust the promised amount of consideration for the effects of a significant financing component, if, at contract inception, the Company expects the period between the time when the Company transfers a promised good or service to the customer and the time when the customer pays for that good or service will be one year or less.
The Company exercises judgment in determining the timing of revenue by analyzing the point in time or the period over which the customer has the ability to direct the use of and obtain substantially all of the remaining benefits of the performance obligation. Typically, over-time revenue recognition is based on the utilization of an input measure used to measure progress, such as costs incurred to date relative to total estimated costs. Changes in total estimated costs are recognized using the cumulative catch-up method of accounting which recognize the cumulative effect of the changes on current and prior periods in the current period. Accordingly, the effect of the changes on future periods of contract performance is recognized as if the revised estimate had been the original estimate. Provisions for estimated losses on uncompleted contracts are made in the period in which such losses are first determined. A significant change in an estimate on one or more contract could have a material effect on the Company’s consolidated financial position, results from operations, or cash flows. However, there were no significant changes in estimated contract costs for the Successor Period of October 20, 2021 through December 31, 2021, the Predecessor Periods of July 1, 2021 through October 19, 2021 and the fiscal year ended June 30, 2021.
If a performance obligation does not qualify for over-time revenue recognition, revenue is then recognized at the point-in-time in which control of the distinct good or service is transferred to the customer, typically based upon the terms of delivery.
Certain of the Company’s products are sold through distributors and third-party sales representatives under standard agreements whereby distributors purchase products from the Company and resell them to customers. These agreements give distributors the right to sell the Company’s products within certain territories and establish minimum order requirements. These arrangements do not provide stock rotation or price protection rights and do not contain extended payment terms. Rights of return are limited to repair or replacement of delivered products that are defective or fail to meet the Company’s published specifications. Provisions for these warranty costs are recognized in the same period that the related revenue is recorded similar to other assurance-type warranties.
Revenue derived from passive dosimetry and analytical services is of a subscription nature and is provided to customers on an agreed-upon recurring monthly, quarterly or annual basis. Services are provided to the customer via passive dosimeter badges that the Company supplies to customer personnel. Depending on the type of badge utilized, either customers return the used badges to the Company for analysis, or they obtain the analysis directly via a self-service web portal. The Company believes that badge production, badge wearing, badge analysis and report preparation are not individually distinct and therefore a single performance obligation recognized over time. Revenue is recognized ratably over the service period as the service is continuous, and no other discernible pattern of recognition is evident. Many customers pay for these measuring and monitoring services in advance. The amounts are recorded as deferred contract revenue in the consolidated balance sheets and represent customer deposits invoiced in advance for services to be rendered over the service period, net of a reserve for estimated cancellations.
Payment terms for shipments to end-users are generally net 30 days. Payment terms for distributor shipments may range from 30 to 90 days. Service arrangements commonly call for payments in advance of performing the work (e.g., extended warranty and service contracts), upon completion of contract milestones (e.g., custom development manufacturing), or a combination of each.
The Company’s costs to obtain contracts are typically comprised of sales commissions. A majority of these costs relate to revenue that is recognized over a period that is less than one year and as such, the Company has elected a practical expedient under ASC 606 to expense these costs as incurred.
Remaining Performance Obligations
The remaining performance obligations for all open contracts as of December 31, 2021 include assembly, delivery, installation, and trainings. The aggregate amount of the transaction price allocated to the remaining performance obligations for all open customer contracts was approximately $747.5 million and $715.8 million as of December 31, 2021 and June 30, 2021, respectively. As of December 31, 2021 the Company expects to recognize approximately 45%, 20%, and 17% of the remaining performance obligations as revenue during the fiscal years 2022, 2023 and 2024, respectively.
Disaggregation of Revenues
A disaggregation of the Company’s revenues by segment, geographic region, timing of revenue recognition and product category is provided in Note 16, Segment Information.
ASC 605
Prior to July 1, 2019, the Company recognized revenue from sales contracts when there was persuasive evidence of an arrangement, product delivery had occurred or services had been provided, the sales price was fixed or determinable and collectability was reasonably assured. For sales contracts that contain customer-specific acceptance provisions, revenue and the related costs were deferred until the customer had indicated successful completion of site acceptance tests or the Company had otherwise determined that all customer-specific acceptance criteria had been met. Where the Company performed detailed factory acceptance testing on completed products, which, in some instances, was sufficiently extensive and reliable to demonstrate that its products meet the customer-specified objective acceptance criteria set forth in the related sales arrangements. In such instances, the Company recognized revenue based on delivery terms and prior to the receipt of notification of formal acceptance from the customer.
The Company combined a group of contracts as one project if they are closely related and were in substance, part of a single project with an overall project margin. The Company segmented a contract into several projects when they were of different business substance, for example, with different business negotiation, solutions, implementation plans, and margins.
The Company evaluated each deliverable in an arrangement to determine whether they represented separate units of accounting. A deliverable constituted a separate unit of accounting when it had stand-alone value, and for an arrangement that included a general right of return relative to the delivered products or services, when delivery or performance of the undelivered product or service was considered probable and is substantially controlled by the Company. The Company considers a deliverable to have stand-alone value if the product or service is sold separately by the Company or another vendor or could be resold by the customer on a stand-alone basis at an amount that would substantially recover the original purchase price. Further, the revenue arrangements generally do not include a general right of return relative to the delivered products. Where the aforementioned criteria for a separate unit of accounting are not met, the deliverable is combined with the undelivered element(s) and treated as a single unit of accounting for the purposes of allocation of the arrangement consideration and revenue recognition.
When a sales arrangement contains multiple units of accounting, the Company allocates the total arrangement consideration to each separable element of an arrangement based upon the relative selling price of each element. Allocation of the consideration is determined at arrangement inception based on each unit’s relative selling price, which is determined based on a selling price hierarchy. The relative selling price for a deliverable is based on its vendor-specific objective evidence (“VSOE”) if available, third-party evidence (“TPE”) if VSOE is not available, or estimated selling price if neither VSOE nor TPE is available. The Company then recognizes revenue on each deliverable in accordance with its policies for product and service revenue recognition. The Company is not typically able to determine VSOE or TPE and therefore uses estimated selling prices to allocate revenue between the elements of the arrangement. The Company establishes its best estimate of the selling price considering multiple factors, including, but not limited to, pricing practices in different
geographies and through different sales channels, costs and margin objectives, competitive pricing strategies and general market conditions.
The Company limits the amount of revenue recognition for delivered elements to the amount that is not contingent on the future delivery of products or services or future performance obligations or subject to customer-specific cancellation rights.
For all arrangements, amounts billed to a customer related to shipping and handling are classified as revenue while all costs incurred by the Company for shipping and handling are classified as cost of revenue. Provisions and allowances for discounts to customers, estimated sales returns, service cancellations, and other adjustments are provided for in the same period that the related revenue is recorded.
Certain of the Company’s products are sold through distributors and third-party sales representatives under standard agreements whereby distributors purchase products from the Company and resell them to customers. These agreements give distributors the right to sell the Company’s products within certain territories and establish minimum order requirements. These arrangements do not provide stock rotation or price protection rights and do not contain extended payment terms. Rights of return are limited to repair or replacement of delivered products that are defective or fail to meet the Company’s published specifications. Provisions for these warranty costs are recognized in the same period that the related revenue is recorded.
Revenue from certain fixed-price contracts that involve customization of equipment to customer specifications is recorded using a percentage-of-completion method measured on the cost-to-cost basis. Contract costs include all direct materials and labor costs, as well as indirect costs related to contract performance. Changes in job performance, job conditions, and estimated profitability result in revisions to costs and revenue and are recognized in the period in which the revisions are determined. Provisions for estimated losses on uncompleted contracts are made in the period in which such losses are first determined. Revenue earned in excess of billings on contracts in progress is classified in the consolidated balance sheet as a current asset and included in costs in excess of billings on uncompleted contracts. Amounts billed in excess of revenue earned are classified as a current liability and included in deferred contract revenue.
Revenue derived from passive dosimetry and analytical services is of a subscription nature and is provided to customers on an agreed-upon recurring monthly, quarterly or annual basis. Services are provided to the customer via passive dosimeter badges that the Company supplies to customer personnel. Depending on the type of badge utilized, either customers return the used badges to the Company for analysis, or they obtain the analysis directly via a self-service web portal. The Company believes that badge production, badge wearing, badge analysis and report preparation are all integral to the benefit that the Company provides to its customers and, therefore, the service period is defined as the period over which all of these services are provided. Revenue is recognized on a straight-line basis over the service period as the service is continuous, and no other discernible pattern of recognition is evident. Many customers pay for these measuring and monitoring services in advance. The amounts are recorded as deferred contract revenue in the consolidated balance sheets and represent customer deposits invoiced in advance for services to be rendered over the service period, net of a reserve for estimated cancellations.
Pertinent to ASC 606 and 605
The Company sells its products and services mainly to large, private and governmental organizations in the Americas, Europe, the Middle East and Asia Pacific regions. The Company performs ongoing evaluations of its customers’ financial condition and limits the amount of credit extended when deemed necessary. The Company generally does not require its customers to provide collateral or other security to support accounts receivable.
No customer represented more than 10% of consolidated revenue for any of the periods and fiscal years presented.
Contract Balances
Revenue earned in excess of billings on contracts in progress (contract assets) are classified in the consolidated balance sheet as a current asset and included in costs in excess of billings on uncompleted contracts. Amounts billed in excess of revenue earned (contract liabilities) are included in deferred contract revenue. For more information, see Note 3, Contracts in Progress.
Selling, General, and Administrative
The Company’s selling, general and administrative expenses consist of direct and indirect costs related to sales and corporate personnel, facilities, professional services, amortization of intangible assets, share-based compensation, and other operating activities.
Advertising Costs
Advertising costs, which the Company expenses when incurred, were approximately $0.4 million, $0.4 million, $0.9 million, $0.9 million, and $0.8 million for the Successor Period from October 20, 2021 through December 31, 2021, the Predecessor Periods from July 1, 2021 through October 19, 2021 and the fiscal years ended June 30, 2021, June 30, 2020, and June 30, 2019 respectively. Trade show costs were approximately $0.5 million, $0.7 million, $0.3 million, $0.6 million, and $0.7 million for the Successor Period from October 20, 2021 through December 31, 2021, the Predecessor Periods from July 1, 2021 through October 19, 2021 and the fiscal years ended June 30, 2021, June 30, 2020, and June 30, 2019 respectively.
Research and Development
Research and development expenses include costs of developing new products and processes, as well as non-project specific design and engineering costs. Research and development costs are expensed as incurred. Development costs related to software incorporated in the Company’s products are not material.
Warrant Liability
As of December 31, 2021, the Company had outstanding warrants to purchase up to 27,249,979 shares of Class A common stock. The Company accounts for the warrants in accordance with the guidance contained in ASC 815, “Derivatives and Hedging”, under which the warrants do not meet the criteria for equity treatment and must be recorded as derivative liabilities. Accordingly, the Company classifies the warrants as liabilities at their fair value and adjusts the warrants to fair value at each reporting period. This liability is subject to re-measurement at each balance sheet date until the warrants are exercised or expire, and any change in fair value is recognized in the Company’s statement of operations. The fair value of the Public Warrants issued in connection with GSAH's initial public offering has been measured based on the listed market price of such Public Warrants. As the transfer of Private Placement Warrants to anyone who is not a permitted transferee would result in the Private Placement Warrants having substantially the same terms as the Public Warrants, we determined that the fair value of each Private Placement Warrant is equivalent to that of each Public Warrant. The determination of the fair value of the warrant liability may be subject to change as more current information becomes available and accordingly the actual results could differ significantly. Derivative warrant liabilities are classified as non-current liabilities as their liquidation is not reasonably expected to require the use of current assets or require the creation of current liabilities. See Note 17, Fair Value Measurements.
Derivative Activities
The Company uses certain derivative financial instruments to help manage its risk or exposure to changes in interest rates in relation to variable rate debt and foreign currency exchange rate fluctuations. The Company records these derivatives at fair value in the balance sheet as either an asset or a liability and any changes in fair value are recognized in earnings as incurred.
Prior to July 1, 2017, the Company entered into an interest rate swap cap agreement, which had an initial notional value of $135.0 million, a cap of 2.0% and expired September 2019. In addition, in fiscal 2018, the Company executed an interest rate swap agreement that has a fixed notional value of $205.0 million and expired in March 2020. In March 2020, the Company executed an interest rate cap agreement for a fixed notional value of $542.0 million with a 2% strike price. The agreement was cancelled in November 2021. There was no notional amount for any instruments at December 31, 2021 and June 30, 2021.
The Company recorded an aggregate net income (loss) of $0.8 million, and $(0.8) million in interest expense in the consolidated statements of operations for the fiscal year ended June 30, 2020 and 2019, respectively, related to these interest rate agreements. No expense related to these interest rate agreements were recognized for the Successor Period of October 20, 2021 through December 31, 2021 and the Predecessor periods of July 1, 2021 through October 19, 2021 and the year ended June 30, 2021.
Stock-Based Compensation Awards
The Company adopted and obtained stockholder approval at its special meeting of the stockholders on October 19, 2021 of the 2021 Omnibus Incentive Plan (the “2021 Plan”). The purpose of the 2021 Plan is to motivate and reward employees
and other individuals to perform at their highest level and contribute significantly to the success of the Company. The 2021 Plan is an omnibus plan that may provide these incentives through grant of stock options, stock appreciation rights, restricted stock, restricted stock units, performance awards, other cash-based awards and other stock-based awards to employees, directors, or consultants of the Company. See Note 14, Stock-based Compensation, for further information on this plan.
Stock-based compensation is rewarded to employees and directors of the Company and accounted for in accordance with ASC 718, "Compensation—Stock Compensation". Stock-based compensation expense is recognized for equity awards over the vesting period based on their grant-date fair value. During the Successor Period, the Company uses various forms of long-term incentives including, but not limited to Restricted Stock Units (“RSUs”) and Performance-based RSUs (“PSUs”), provided that the issuance of such stock options was contingent upon the Company filing a registration statement on Form S-8 with the SEC, which occurred on December 27, 2021. The grant date fair value of the PSUs is determined using a Monte Carlo simulation model. The grant date fair value of the RSUs is determined using the closing price of the Company’s Class A common stock price on the grant date. Stock-based compensation expense is included within the same financial statement caption where the recipient’s other compensation is reported. The Company accounts for forfeitures as they occur.
In conjunction with entering into the Business Combination Agreement, on June 17, 2021 the Sponsor issued membership interests to certain Mirion employees and the current Chairman of the Board of Mirion (collectively, the "Profits Interests"). The Profits Interests are subject to service and performance vesting conditions and do not fully vest until all of the applicable conditions are satisfied. In addition, the Profits Interests are subject to certain forfeiture conditions. Accordingly, these awards have been treated as stock based compensation under ASC 718. The grant date fair value of the Profits Interests is based upon a valuation model using Monte Carlo simulations. As the Profits Interests included the completion of the Business Combination as a vesting condition, the expense that accumulated prior to the Business Combination was recorded on the last day of the Predecessor Period and the remainder is recorded over the future vesting period.
Prior to the Business Combination, the Company accounted for share-based compensation related to restricted stock awards granted to certain employees by recognizing the grant date fair value of the awards over the requisite service period, which is equal to the vesting period. The Company had the option to buy back the unvested awards upon termination of employment at the lesser of the original issuance price paid by employees or the fair value of the shares on the buy-back date. The Company estimated the value of the restricted stock awards by using the Black-Scholes option valuation model, which requires the use of certain subjective assumptions. Significant assumptions include management’s estimates of the estimated stock price volatility, the expected life of the awards and related employee forfeiture rates.
For more information see Note 14, Stock-based Compensation.
Accounting for Income Taxes
The Company accounts for income taxes and the related accounts under the asset and liability method. Deferred tax assets and liabilities are recognized for the expected tax consequences of temporary differences between the tax bases of assets and liabilities and their reported amounts. Valuation allowances are recorded to reduce deferred tax assets to the amount that will more likely than not be realized. The Company classifies all deferred tax assets and liabilities, and any related valuation allowance, as non-current in the consolidated balance sheet.
The Company accounts for uncertainty in income taxes using a two-step approach to recognizing and measuring uncertain tax positions. The first step is to evaluate the tax position for recognition by determining if the weight of available evidence indicates that it is more likely than not that the position will be sustained on audit, including resolution of related appeals or litigation processes, if any. The second step is to measure the tax benefit as the largest amount that is more than 50% likely of being realized upon settlement. The Company classifies the liability for unrecognized tax benefits as current in the balance sheet, to the extent that the Company anticipates payment or receipt of cash within one year. Interest and penalties related to uncertain tax positions are recognized in the provision for income taxes.
Defined Benefit Pension Plans and Other Employee Benefits
The Company has defined benefit pension plans that cover certain of its employees in France, Japan, and Germany. The Company also has a post-retirement plan that provides for the reimbursement of a portion of medical and life insurance premiums for certain retirees and eligible dependents in the United States. Plan liabilities are revalued annually based on assumptions relating to the discount rates used to measure future obligations and expenses, salary-scale inflation rates, mortality and other assumptions. The selection of assumptions is based on historical trends and known economic and market conditions at the time of valuation; however, actual results may differ from the Company’s estimates.
Foreign Currency Translation
Local currency is the functional currency for substantially all of the Company’s foreign operations. Assets and liabilities of foreign operations are translated into U.S. dollars using the exchange rates in effect at the balance sheet reporting date, while income and expenses are translated at the average monthly exchange rates during the period. We record gains and losses from the translation of financial statements in foreign currencies into U.S. dollars in other comprehensive income. The income tax effect of currency translation adjustments related to foreign subsidiaries that are not considered indefinitely reinvested is recorded as a component of deferred taxes with an offset to other comprehensive income. We record gains and losses from changes in exchange rates on transactions denominated in currencies other than each reporting location’s functional currency in the consolidated statements of operations for each period.
Concentrations of Risk
Financial instruments that are potentially subject to concentration of credit risk consist primarily of cash and cash equivalents and accounts receivable. The Company maintains cash in bank deposit accounts that, at times, may exceed the insured limits of the local country. The Company has not experienced any losses in such accounts.
The Company sells its products and services mainly to large, private and governmental organizations in the Americas, Europe, the Middle East and Asia Pacific regions. The Company performs ongoing evaluations of its customers’ financial condition and limits the amount of credit extended when deemed necessary. The Company generally does not require its customers to provide collateral or other security to support accounts receivable. As of December 31, 2021, June 30, 2021, and June 30, 2020, no customer accounted for more than 10% of the accounts receivable balance.
Loss Per Share
Net loss per share of common stock is computed using the two-class method required for multiple classes of common stock and participating securities based upon their respective rights to receive dividends as if all income for the period has been distributed.
Net loss per share of common stock is computed using the two-class method required for multiple classes of common stock and participating securities based upon their respective rights to receive dividends as if all income for the period has been distributed. Basic loss per share is computed by dividing loss available to common stockholders by the weighted average number of common shares outstanding, adjusted for the outstanding non-vested shares. Diluted loss per share is computed by giving effect to all potentially dilutive securities outstanding for the period using the treasury stock method or the if-converted method based on the nature of such securities. For periods in which the Company reports net losses, diluted net loss per common share attributable to common stockholders is the same as basic net loss per common share attributable to common stockholders, because potentially dilutive common shares are not assumed to have been issued if their effect is anti-dilutive.
Successor Period
Upon the closing of the Business Combination, the following classes of stock were considered in the loss per share calculation.
Class A Common Stock
Holders of shares of our Class A common stock are entitled to one vote for each share held of record on all matters on which stockholders are entitled to vote generally, including the election or removal of directors. The holders of our Class A common stock do not have cumulative voting rights in the election of directors. Holders of shares of our Class A common stock are entitled to receive dividends when and if declared by our Board out of funds legally available therefor, subject to any statutory or contractual restrictions on the payment of dividends and to any restrictions on the payment of dividends imposed by the terms of any outstanding preferred stock. Upon our liquidation, dissolution or winding up and after payment in full of all amounts required to be paid to creditors and to the holders of preferred stock having liquidation preferences, if any, the holders of shares of our Class A common stock will be entitled to receive pro rata our remaining assets available for distribution. Class A common stock issued and outstanding is included in the Company’s basic loss per share calculation.
Class B Common Stock
Holders of shares of our Class B common stock also hold shares of IntermediateCo Class B common stock on a one-to-one basis (the "paired interests"). Holders of shares of our Class B common stock are entitled to one vote for each share held of record on all matters on which stockholders are entitled to vote generally, including the election or removal of directors. If
at any time the ratio at which shares of IntermediateCo Class B common stock are redeemable or exchangeable for shares of our Class A common stock changes from a one-for-one basis, the number of votes to which our Class B common stockholders are entitled will be adjusted accordingly. The holders of our Class B common stock do not have cumulative voting rights in the election of directors. Except for transfers to us pursuant to the IntermediateCo Charter or to certain permitted transferees set forth in our Charter, the shares of our Class B common stock and corresponding shares of IntermediateCo Class B common stock may not be sold, transferred or otherwise disposed of.
Holders of shares of our Class B common stock are not entitled to economic interests in us or to receive dividends or to receive a distribution upon our liquidation or winding up. However, if IntermediateCo makes distributions to us other than solely with respect to our Class A common stock, the holders of shares of IntermediateCo Class B common stock will be entitled to receive distributions pro rata in accordance with the percentages of their respective shares of IntermediateCo Class B common stock.
Our shares of Class B common stock are excluded from the calculation of basic and diluted earnings per share because such shares have voting rights but no economic interest in the Company.
Warrants
As described above, the Company has outstanding warrants to purchase up to 27,249,979 shares of Class A common stock. One whole warrant entitles the holder thereof to purchase one share of Mirion Class A common stock at a price of $11.50 per share. The Company’s warrants are not included in the Company’s calculation of basic loss per share but excluded from the calculation of diluted loss per share because their inclusion would be anti-dilutive.
Founder Shares
Founder shares are shares of Class A common stock subject to certain vesting events and forfeiture if a required vesting event does not occur within five years of the closing of the Business Combination. The founder shares are subject to vesting in three equal tranches, based on the volume-weighted average price of the Class A common stock being greater than or equal to $12.00, $14.00 and $16.00 per share for any 20 trading days in any 30 consecutive trading day period. Holders of the founder shares are entitled to vote such founder shares and receive dividends and other distributions with respect to such founder shares prior to vesting, but such dividends and other distributions with respect to unvested founder shares will be set aside by the Company and shall only be paid to the holders of the founder shares upon the vesting of such founder shares.
As the holders of the founder shares are not entitled to participate in earnings unless the vesting conditions are met, the founders shares have been excluded from the calculation of basic earnings per share. The founders shares are also excluded from the calculation of diluted earnings per share because their inclusion would be anti-dilutive.
Predecessor Period
In the Predecessor Periods presented, the rights, including the liquidation, dividend rights, sharing of losses, and voting rights of the A Ordinary Shares and B Ordinary Shares of Mirion TopCo were identical. As the rights of both classes of shares were identical, the undistributed earnings are allocated on a proportionate basis and the resulting net loss per share attributed to common stockholders is therefore the same for A Ordinary Shares and B Ordinary Shares on an individual or combined basis.
The Company’s participating securities include the Company’s non-vested A Ordinary Shares, as the holders are entitled to non-forfeitable dividend rights in the event a dividend were paid on common stock. The holders of non-vested A Ordinary Shares did not have a contractual obligation to share in losses.
The rights, including the liquidation, dividend rights, sharing of losses, and voting rights of the A Ordinary Shares and B Ordinary Shares are identical. As the rights of both classes of shares were identical, the undistributed earnings were allocated on a proportionate basis and the resulting net loss per share attributed to common stockholders was therefore the same for A Ordinary Shares and B Ordinary Shares on an individual or combined basis.
Basic loss per share is computed by dividing loss available to shareholders by the weighted average number of common shares outstanding, adjusted for the outstanding non-vested shares. Diluted loss per share is computed by giving effect to all potentially dilutive securities outstanding for the period using the treasury stock method or the if-converted method based on the nature of such securities. For periods in which the Company reports net losses, diluted net loss per common share attributable to shareholders is the same as basic net loss per ordinary share attributable to shareholders, because potentially dilutive common shares are not assumed to have been issued if their effect is anti-dilutive.
Recent Accounting Pronouncements
Accounting Guidance Issued But Not Yet Adopted
In March 2020, the FASB issued ASU 2020-04 “Reference Rate Reform (Topic 848): Facilitation of the Effects of Reference Rate Reform on Financial Reporting”. ASU 2020-04 provides temporary optional expedients and exceptions for applying GAAP guidance on contract modifications and hedge accounting to ease the financial reporting burdens of the expected market transition from the LIBOR and other interbank offered rates to alternative reference rates, such as the Secured Overnight Financing Rate. For all entities, ASU 2020-04 can be adopted after its issuance date through December 31, 2022. The Company is currently evaluating the impact of this ASU.
Recently Adopted Accounting Guidance
In October 2021, the FASB issued ASU No. 2021-08, "Business Combinations (Topic 805): Accounting for Contract Assets and Contract Liabilities from Contracts with Customers”, which requires entities to recognize and measure contract assets and contract liabilities acquired in a business combination in accordance with ASC 606, Revenue from Contracts with Customers. The guidance will be effective for annual reporting periods beginning after December 15, 2022, including interim periods therein. Early adoption is permitted, including in an interim period for which the financial statements have not been issued. If early adopting in an interim period, the Company is required to apply the amendments to all prior business combinations that have occurred since the beginning of the fiscal year that includes the interim period of application. The Company adopted ASU 2021-08 in the quarter ended December 31, 2021, and retroactively applied the new guidance to all business combinations that occurred since the beginning of the fiscal year. The adoption of ASU 2021-08 resulted in no adjustments being required to the deferred revenue acquired with business combinations that occurred since the beginning of the fiscal year.
In August 2018, the FASB issued ASU 2018-13, "Fair Value Measurement (Topic 820): Disclosure Framework — Changes to the Disclosure Requirements for Fair Value Measurement". ASU 2018-13 eliminates, adds and modifies certain disclosure requirements for fair value measurements as part of the FASB’s disclosure framework project. For all entities, ASU 2018-13 is effective for annual and interim reporting periods beginning after December 15, 2019. Certain amendments must be applied prospectively while others are to be applied on a retrospective basis to all periods presented. The Company adopted this guidance as of July 1, 2020, and it did not have a material impact on the consolidated financial statements or related disclosures.
In August 2018, the FASB issued ASU 2018-15, "Intangibles – Goodwill and Other – Internal Use Software (Subtopic 350-40): Customer’s Accounting for Implementation Costs Incurred in a Cloud Computing Arrangement that is a Service Contract", which amended the accounting for implementation, setup and other upfront costs for entities that are a customer in a hosting arrangement that is a service contract. ASU 2018-15 was effective for public business entities for fiscal years beginning after December 15, 2019, and interim periods within those fiscal years. For all other entities, including emerging growth companies, the amendments are effective for fiscal years beginning after December 15, 2020, and interim periods within fiscal years beginning after December 15, 2021. Early adoption is permitted, including adoption in any interim period for which financial statements have not been issued. Application of the amendments can be applied either retrospectively or prospectively to all implementation costs incurred after the date of adoption. The Company adopted this guidance as of July 1, 2021, prospectively, which did not have a material impact on our consolidated financial statements
In June 2016, the FASB issued ASU 2016-13, "Financial Instruments – Credit Losses (Topic 326): Measurement of Credit Losses on Financial Instruments", which replaced the incurred loss impairment methodology in current GAAP with a methodology that reflects expected credit losses and requires consideration of a broader range of reasonable and supportable information to inform credit loss estimates. For trade and other receivables, loans, and other financial instruments, we will be required to use a forward-looking expected loss model rather than the incurred loss model for recognizing credit losses which reflects losses that are probable. Credit losses relating to available-for-sale debt securities will also be recorded through an allowance for credit losses rather than as a reduction in the amortized cost basis of the securities. Application of the amendments is through a cumulative-effect adjustment to retained earnings as of the effective date. The Company adopted this guidance as of July 1, 2021, prospectively, which did not have a material impact on our consolidated financial statements.
In February 2016, the FASB issued ASU 2016-02, "Leases (Topic 842)", which, among other things, requires an entity to recognize a right-of-use asset and a lease liability on the balance for substantially all leases, including operating leases. Expanded disclosures with additional qualitative and quantitative information are also required. ASC 2016-02 and its related amendments were effective for public companies with interim and annual reporting periods beginning after
December 15, 2018, and for non-public companies, including emerging growth companies, with interim and annual reporting periods beginning after December 15, 2021. Early adoption was permitted.
The Company adopted ASU 2016-02 and its amendments as of July 1, 2021, using the modified retrospective method and under such method no recast of prior-period financial statements was presented. The Company elected the package of practical expedients permitted under the transition guidance, which allowed the Company to carryforward its historical assessments of: (1) whether contracts are or contain leases, (2) lease classification and (3) initial direct costs. In addition, the Company did not elect the hindsight practical expedient to determine the reasonably certain lease term for existing leases. The Company elected a policy of not applying the recognition requirements to leases with a term of less than twelve months. The Company elected to account for lease and non-lease components as a combined single lease component.
The Company’s adoption of the standard resulted in the recognition of right-of-use ("ROU") assets of $47.2 million (net of deferred rent and impairment upon adoption) with corresponding operating lease liabilities of $52.1 million. The Company's adoption of the standard also resulted in a cumulative-effect adjustment to increase accumulated deficit by $2.9 million, net of taxes, as of July 1, 2021. Refer to Note 9, Leased Assets, for further detail. The standard did not materially impact the Company’s consolidated net income or liquidity and did not have an impact on debt-covenant compliance under the Company’s debt agreements. Refer to Note 8, Borrowings, for further detail.
2. Business Combinations and Acquisitions
Business Combination
On October 20, 2021, Mirion Technologies, Inc. consummated its previously announced Business Combination pursuant to the Business Combination Agreement.
The aggregate Business Combination Consideration paid by the Company to the Sellers in connection with the consummation of the Business Combination was $1.3 billion in cash, 30,401,902 newly issued shares of Class A common stock and 8,560,540 newly issued shares of the Company’s Class B common stock. The Sellers receiving shares of Class B common stock also received one share of IntermediateCo Class B common stock per share of Class B common stock as a paired interest. Each of the shares of Class A common stock and each paired interest were valued at $10.00 per share for purposes of determining the aggregate number of shares issued to the Sellers.
The Business Combination is being accounted for under ASC 805, "Business Combinations". GSAH was determined to be the accounting acquirer. Mirion TopCo constitutes a business in accordance with ASC 805 and the Business Combination constitutes a change in control. Accordingly, the Business Combination is being accounted for using the acquisition method. Under this method of accounting, Mirion TopCo is treated as the “acquired” company for financial reporting purposes and our net assets are stated at fair value, with goodwill or other intangible assets recorded.
As a result of the Business Combination, the Company’s financial statement presentation distinguishes Mirion TopCo as the “Predecessor” through the Closing Date. The Company, which includes the combination of GSAH and Mirion TopCo subsequent to the Business Combination, is the “Successor” for periods after the Closing Date. As a result of the application of the acquisition method of accounting in the Successor Period, the financial statements for the Successor Period are presented on a full step-up basis as a result of the Business Combination, and are therefore not comparable to the financial statements of the Predecessor Periods that are not presented on the same full step-up basis due to the Business Combination.
The following table summarizes the consideration transferred by GSAH:
| | | | | |
| Cash consideration paid by GSAH | $ | 1,310.0 | |
| Cash repayment of existing Mirion TopCo third-party debt | 903.6 | |
| Reimbursement of Mirion TopCo transaction costs | 11.7 | |
| Cash consideration paid by GSAH | $ | 2,225.3 | |
Shares issued to Mirion TopCo sellers at fair value (1) | 407.0 | |
| Total consideration transferred | $ | 2,632.3 | |
(1)A total of 30,401,902 shares of Class A common stock were issued to the Sellers at fair value and recognition of noncontrolling interests for 8,560,540 shares Class B common stock at the Closing.
The following table summarizes the provisional total business enterprise value, comprised of the preliminary fair value of net assets acquired for the Business Combination. The estimated fair values of all assets acquired and liabilities assumed in the acquisition are provisional and may be revised as a result of additional information obtained during the measurement period of up to one year from the acquisition date, including but not limited to valuation of tax accounts, property, plant and equipment and intangible assets.
| | | | | | | | | | | | | | | | | | | | | | | |
| Mirion TopCo |
| Date of acquisition | October 20, 2021 |
| Segment | Medical | | Industrial | | Corporate | | Total |
| Goodwill (1) | $ | 675.2 | | | $ | 963.8 | | | $ | — | | | $ | 1,639.0 | |
| Customer relationships (2) | 152.7 | | | 186.1 | | | — | | | 338.8 | |
| Developed technology (3) | 66.3 | | | 168.3 | | | — | | | 234.6 | |
| Tradenames (4) | 36.8 | | | 63.7 | | | — | | | 100.5 | |
| Distributor relationships (5) | 52.5 | | | 8.6 | | | — | | | 61.1 | |
| Backlog (6) | 17.7 | | | 63.8 | | | — | | | 81.5 | |
| Non-compete agreements (7) | 4.5 | | | — | | | — | | | 4.5 | |
| Amortizable intangible assets | $ | 330.5 | | | $ | 490.5 | | | $ | — | | | $ | 821.0 | |
| Cash | 7.8 | | | 39.5 | | | 54.6 | | | 101.9 | |
| Accounts receivable | 44.0 | | | 70.3 | | | — | | | 114.3 | |
| Cost in excess of billings | — | | | 63.6 | | | — | | | 63.6 | |
| Inventory | 25.1 | | | 119.5 | | | — | | | 144.6 | |
| Property, Plant and Equipment | 52.6 | | | 72.7 | | | 1.1 | | | 126.4 | |
| Other current and non-current assets | 5.8 | | | 13.2 | | | 5.3 | | | 24.3 | |
| Right of use assets | 22.3 | | | 20.1 | | | 0.9 | | | 43.3 | |
| Other non-current assets | 8.0 | | | 9.0 | | | — | | | 17.0 | |
| Current liabilities | (31.9) | | | (82.7) | | | (33.7) | | | (148.3) | |
| Current lease liability | (4.1) | | | (4.4) | | | (0.3) | | | (8.8) | |
| Deferred contract revenue | (34.7) | | | (24.2) | | | — | | | (58.9) | |
| Notes payable assumed | (1.8) | | | (1.1) | | | — | | | (2.9) | |
| Other long-term liabilities | (70.6) | | | (147.7) | | | (23.8) | | | (242.1) | |
| Minority interest | — | | | (2.0) | | | (0.1) | | | (2.1) | |
| Net tangible assets acquired | $ | 22.5 | | | $ | 145.8 | | | $ | 4.0 | | | $ | 172.3 | |
| Purchase consideration | | | | | | | 2,632.3 | |
| Less: cash acquired | | | | | | | (101.9) | |
| GAAP purchase consideration, net of cash acquired | | | | | | | $ | 2,530.4 | |
(1)The goodwill of $1,639.0 million represents the excess of the gross consideration transferred over the fair value of the underlying net tangible and identifiable intangible assets acquired and liabilities assumed. Qualitative factors that contribute to the recognition of goodwill include certain intangible assets that are not recognized as separate identifiable intangible assets apart from goodwill. Intangible assets not recognized apart from goodwill consist primarily of the strong market position and the assembled workforce of Mirion TopCo. A portion of the goodwill recognized is expected to be deductible for income tax purposes. The purchase price allocation has not been finalized. We expect to finalize the valuation report and complete the purchase price allocation no later than one-year from the acquisition date.
(2)The useful life for customer relationships ranges from 6 to 13 years.
(3)The useful life for developed technology ranges from 5 to 16 years.
(4)The useful life for tradenames is 10 years.
(5)The useful life for distributor relationships ranges from 7 to 13 years.
(6)The useful life for backlog ranges from 1 to 4 years.
(7)The useful life for non-compete agreements is 1 year.
In connection with the acquisitions of Mirion TopCo, the Company incurred approximately $2.2 million and $26.2 million of transaction expenses for the Successor Period from October 20, 2021 through December 31, 2021 and the Predecessor Period from July 1, 2021 through October 19, 2021, respectively.
Unaudited Pro Forma Financial Information
The following unaudited pro forma financial information presents the Company’s results of operations for the years ended December 31, 2021 and June 30, 2021 to illustrate the estimated effects of the acquisition of Mirion as if it had occurred on July 1, 2020. The pro forma financial information is presented for comparative purposes only and is not necessarily indicative of the Company’s operating results that may have actually occurred had the acquisition of Mirion had been completed on July 1, 2020. The unaudited pro forma financial information does not reflect the expected realization of any anticipated cost savings, operating efficiencies, or other synergies that may have been associated with the acquisition.
| | | | | | | | | | | | | | | | | | | | |
| (amounts in millions) | Successor | | | Predecessor |
| | From
October 20, 2021
through
December 31, 2021 | | | From July 1, 2021 through
October 19, 2021 | | Fiscal Year Ended June 30, 2021 |
| Total revenues | $ | 154.1 | | | | $ | 168.0 | | | $ | 611.6 | |
| Net income (loss) | $ | (5.2) | | | | $ | (56.3) | | | $ | (192.1) | |
| Net income (loss) attributable to Mirion Technologies, Inc. stockholders | $ | (3.6) | | | | $ | (54.0) | | | $ | (184.2) | |
The unaudited pro forma financial information reflects pro forma adjustments to present the combined pro forma results of operations as if the acquisition had occurred on July 1, 2020 to give effect to certain events the Company believes to be directly attributable to the acquisitions. These pro forma adjustments primarily include:
•A net increase in cost of revenues, depreciation, and amortization expense that would have been recognized due to acquired inventory, property, plant and equipment and intangible assets;
•A decrease in interest expense to reflect the elimination of interest expense on debt assumed settled as of July 1, 2020, and the recognition of interest on new debt issued in conjunction with the acquisition;
•A reduction in expenses for the Successor Period from October 20, 2021 through December 31, 2021 and the Predecessor Period from July 1, 2021 through October 19,2021, and a corresponding increase in the fiscal year ended June 30, 2021, for acquisition-related transaction costs directly attributable to the acquisition;
•A reduction in expenses for the Successor Period from October 20, 2021 through December 31, 2021 and the Predecessor Period from July 1, 2021 through October 19, 2021, and a corresponding increase in the fiscal year ended June 30, 2021, for stock-based compensation related to Profits Interests;
•A reversal of gain due to a change in fair value of warrants for the Successor Period from October 20, 2021 through December 31, 2021, and a corresponding gain in fair value of the warrants in the fiscal year ended June 30, 2021;
•A change in income tax expense to reflect the income tax effect of the pro forma adjustments based upon an estimated blended statutory rate of 25%; and
•The attribution of the non-controlling interest for the Class B shares of common stock issued to certain existing Mirion TopCo stockholders.
For the Successor Period ended December 31, 2021 and the Predecessor Periods ended October 19, 2021 and fiscal year ended June 30, 2021, pro forma adjustments directly attributable to the acquisitions include (i) the purchase accounting effect of inventories acquired of $15.8 million, and (ii) transaction costs of $28.4 million.
Acquisitions
The Company continually evaluates potential acquisitions that strategically fit with the Company’s existing portfolio of businesses and as a result, the Company completed a number of acquisitions during the Successor and Predecessor Periods presented.
All acquisitions are accounted for under the acquisition method of accounting, with the related assets acquired and liabilities assumed recorded at fair value. The Company makes an initial allocation of the purchase price at the date of
acquisition based upon its understanding of the fair value of the acquired assets and assumed liabilities. The Company obtains the information used for the purchase price allocation during due diligence and through other sources. In the months after closing, as the Company obtains additional information about the acquired assets and liabilities, including through tangible and intangible asset appraisals, and learns more about the newly acquired business, it is able to refine the estimates of fair value and more accurately allocate the purchase price. The fair values of acquired intangibles are determined based on estimates and assumptions that are deemed reasonable by the Company. Significant assumptions include the discount rates and certain assumptions that form the basis of the forecasted results of the acquired business including revenue, earnings before interest, taxes, depreciation and amortization (“EBITDA”), and growth rates. These assumptions are forward looking and could be affected by future economic and market conditions. The Company engages third-party valuation specialists who review the Company’s critical assumptions and calculations of the fair value of acquired intangible assets in connection with material acquisitions. Only facts and circumstances that existed as of the acquisition date are considered for subsequent adjustment. The Company will make appropriate adjustments to the purchase price allocation prior to completion of the measurement period, as required.
The acquisition of these businesses resulted in the recognition of goodwill in the Company’s Consolidated Financial Statements, which is calculated as the excess of the consideration transferred over the net assets recognized and represents the future economic benefits arising from the other assets acquired that could not be individually identified and separately recognized. The goodwill is not amortized but some portion may be deductible for income tax purposes. This goodwill recorded includes the following:
•The expected synergies and other benefits that we believe will result from combining the operations of the acquired business with the operations of Mirion;
•Any intangible assets that did not qualify for separate recognition, as well as future, yet unidentified projects and products;
•The value of the existing business as an assembled collection of net assets versus if the Company had acquired all of the net assets separately.
Successor Period Acquisitions:
The following briefly describes the Company’s acquisition activity subsequent to the Business Combination and prior to December 31, 2021.
| | | | | | | | | | | | | | | | | | | | |
Year Ended December 31, | | Company Name | | Description of the Business | | Description of the Acquisition |
| 2021 | | CIRS | | Computerized Imaging Reference Systems, Inc. ("CIRS") is a U.S.-based company which specializes in design, development, and commercialization of tissue equivalent medical imaging and radiation therapy phantoms. | | On December 1, 2021, the Company acquired 100% of the equity interest for approximately $55.1 million, subject to final closing statement balances. |
| 2021 | | Safeline | | Safeline Monitors Systems LLC is a U.S.-based provider of dosimetry services which will increase the U.S. footprint of Mirion’s industry-leading dosimetry product offerings. | | On December 1, 2021, the Company acquired 100% of the member equity interest for approximately $1.5 million, which includes a $0.5 million contingent consideration, based on actual revenues from existing customers for 6 months subsequent to the transaction date. |
| 2021 | | CHP | | CHP Dosimetry is a U.S.-based provider of dosimetry services which will increase the U.S. footprint of Mirion’s industry-leading dosimetry product offerings. | | On November 1, 2021, the Company acquired 100% of the assets for approximately $2.5 million, subject to final closing statement balances. |
The following table summarizes the provisional total business enterprise value, comprised of the preliminary fair value of net assets acquired for the CIRS acquisition. The estimated fair values of all assets acquired and liabilities assumed in the acquisition are provisional and may be revised as a result of additional information obtained during the measurement period of up to one year from the acquisition date, including but not limited to valuation of tax accounts, property, plant and equipment and intangible assets.
| | | | | |
| (in millions) | CIRS |
| Date of acquisition | December 1, 2021 |
| Segment | Medical |
| Goodwill | $ | 35.0 | |
| Developed technology (1) | 19.2 | |
| Customer relationships (2) | 1.6 | |
| Tradenames (3) | 0.4 | |
| Backlog (4) | 0.6 | |
| Amortizable intangible assets | $ | 21.8 | |
| Cash | 1.0 | |
| Accounts receivable | 1.6 | |
| Inventory | 2.0 | |
| Property, Plant and Equipment | 0.4 | |
| Operating ROU assets | 3.8 | |
| Current lease liabilities | (0.5) | |
| Other long-term liabilities | (10.0) | |
| Net tangible assets acquired | $ | (1.7) | |
| Purchase consideration | 55.1 | |
| Less: cash acquired | (1.0) | |
| GAAP purchase consideration, net of cash acquired | $ | 54.1 | |
| Acquiree revenue post acquisition through the period ended December 31, 2021 | $ | 1.5 | |
| Acquiree income (loss) from operations post acquisition through the period ended December 31, 2021 | $ | (0.1) | |
(1)The useful life for developed technology is 5 years.
(2)The useful life for customer relationships is 7 years.
(3)The useful life for tradenames is 3 years.
(4)The useful life for backlog is 2 years.
In connection with the acquisitions of CIRS, the Company incurred approximately $0.4 million of transaction expenses for the period ended December 31, 2021.
Predecessor Period Acquisitions:
The following briefly describes the Company’s acquisition activity prior to the Business Combination for the Predecessor Periods ended October 19, 2021 and fiscal years ended June 30, 2021, 2020, and 2019.
| | | | | | | | | | | | | | | | | | | | |
Predecessor Periods ended October 19, 2021 | | Company Name | | Description of the Business | | Description of the Acquisition |
| 2021 | | Dosimetry Badge | | Dosimetry Badge is a U.S.-based provider of dosimetry services which will increase the U.S. footprint of Mirion’s industry-leading dosimetry product offerings. | | On September 1, 2021 the Company acquired 100% of the assets for approximately $1.8 million, which includes a $0.8 million earn-out, based on revenues from existing customers for 12 months subsequent to the transaction date. |
Year Ended June 30, | | Company Name | | Description of the Business | | Description of the Acquisition |
| 2021 | | Sun Nuclear | | Sun Nuclear Corporation (“SNC” or “Sun Nuclear”) is a provider in radiation oncology quality assurance, delivering patient safety solutions for diagnostic imaging and radiation therapy centers around the world. | | On December 18, 2020, the Company acquired 100% of the equity interest for approximately $258.1 million of purchase consideration, net of cash acquired. |
| 2021 | | Dosimetrics | | Dosimetrics is a provider in the development and production of OSL personal radiation dosimeters and dosimetry solutions, including readers, erasers, software, accessories, and automation systems. | | On December 1, 2020, the Company acquired 100% of the equity interest for approximately $3.0 million of purchase consideration, net of cash acquired. |
| 2021 | | Biodex | | Biodex is a manufacturer and distributor of medical devices and related replacement parts for physical and nuclear medicine, as well as medical imaging applications located in the United States. | | On September 1, 2020, the Company acquired 100% of the equity interest for approximately $26.9 million of purchase consideration, net of cash acquired. |
| 2020 | | AWST | | AWST is a provider of calibration and measurement technologies for radiation medicine applications. | | On March 31, 2020, the Company acquired 100% of the equity interest for approximately €24.5 million (or $26.9 million) of purchase consideration. |
| 2020 | | Selmic | | Selmic is an electronic component manufacturer of sensors, modules, and devices serving in automotive, transportation, medical, security, defense, and telecom industries. | | On October 31, 2019, the Company acquired 100% of the equity interest for approximately €9.1 million (or $10.2 million) of purchase consideration. |
| 2020 | | Premium Analyse | | Premium Analyse is a provider in the radioactive gas detection market and measurement of tritium. | | On July 19, 2019, the Company acquired 100% of the equity interest for approximately €7.9 million ($8.9 million) of purchase consideration. |
| 2020 | | Capintec | | Capintec is a provider of calibration and measurement technologies for nuclear medicine applications. Capintec provides solutions for applications in nuclear medicine, nuclear cardiology, oncology, endocrinology, diagnostic radiology, and radiation therapy. | | On July 9, 2019, the Company acquired 100% of the equity interest for approximately $14.5 million of purchase consideration. |
| 2019 | | NRG Dosimetry Services Group | | NRG Dosimetry Services Group is a provider of dosimetry services in the Netherlands. | | On October 31, 2018, the Company acquired 100% of the equity interest for approximately €7.8 million (or $9.1 million) of purchase consideration |
The following summarizes the fair value of assets acquired and liabilities assumed for the Biodex and SNC acquisitions during the year ended June 30, 2021 (in millions):
| | | | | | | | | | | |
| Predecessor |
| Biodex | | SNC |
| Date of acquisition | September 1, 2020 | | December 18, 2020 |
| Segment | Medical | | Medical |
| Goodwill | $ | 11.1 | | | $ | 130.2 | |
| Customer relationships (1) | 2.3 | | | 59.5 | |
| Tradenames (2) | 1.4 | | | 12.0 | |
| Non-Compete Agreements (3) | 0.3 | | | 7.5 | |
| Developed Technology (4) | 2.6 | | | 46.5 | |
| Amortizable intangible assets | $ | 6.6 | | | $ | 125.5 | |
| Cash | 4.1 | | | 18.8 | |
| Accounts receivable | 4.0 | | | 24.0 | |
| Inventory | 6.4 | | | 13.9 | |
| Property, Plant and Equipment | 1.0 | | | 5.9 | |
| Other current and non-current assets | 0.6 | | | 8.0 | |
| Current liabilities | (2.6) | | | (9.3) | |
| Deferred contract revenue | (0.2) | | | (6.5) | |
| Other long-term liabilities | — | | | (33.6) | |
| Net tangible assets acquired | $ | 13.3 | | | $ | 21.2 | |
| Purchase consideration (5) | 31.0 | | | 276.9 | |
| Less: cash acquired | (4.1) | | | (18.8) | |
| GAAP purchase consideration, net of cash acquired | $ | 26.9 | | | $ | 258.1 | |
| Acquiree revenue post acquisition through the period ended June 30, 2021 | $ | 32.6 | | | $ | 48.9 | |
| Acquiree income (loss) from operations post acquisition through the period ended June 30, 2021 | $ | 0.7 | | | $ | (5.5) | |
The following useful lives were used for the initial acquisition and were all reassessed in connection with the Business Combination:
(1)The useful life for customer relationships ranges from 10 to 11 years.
(2)The useful life for tradenames is 7 years.
(3)The useful life for non-compete agreements ranges from 2 to 3 years.
(4)The useful life for developed technology ranges from 7 to 10 years.
(5)Biodex purchase consideration consisted of cash. SNC purchase consideration consisted of $261.9 million cash and $15.0 million of deferred consideration paid in February 2021.
In connection with the acquisition of Sun Nuclear, the Company incurred approximately $1.2 million of transaction expenses for the year ended June 30, 2021.
Unaudited Pro Forma Financial Information
The following unaudited pro forma financial information presents the Company’s results of operations for the years ended June 30, 2021 and June 30, 2020 to illustrate the estimated effects of the acquisitions of Biodex and SNC as if they had occurred on July 1, 2019. The pro forma financial information is presented for comparative purposes only and is not necessarily indicative of the Company’s operating results that may have actually occurred had the acquisitions of Biodex and SNC been completed on July 1, 2019. The unaudited pro forma financial information does not reflect the expected realization of any anticipated cost savings, operating efficiencies, or other synergies that may have been associated with the acquisitions.
| | | | | | | | | | | |
| (amounts in millions) | Years ended June 30, |
| | 2021 | | 2020 |
| Total revenues | $ | 670.9 | | | $ | 598.7 | |
| Net loss | (142.9) | | (239.2) |
| Net loss attributable to Mirion TopCo stockholders | (127.9) | | (158.3) |
The unaudited pro forma financial information reflects pro forma adjustments to present the combined pro forma results of the operations as if the acquisitions had occurred on July 1, 2020 to give effect to certain events the Company believes to be directly attributable to the acquisitions. These pro forma adjustments primarily include:
•A net increase in cost of revenues, depreciation and amortization expense that would have been recognized due to acquired inventory, property, plant and equipment and intangible assets;
•An increase to interest expense to reflect the additional borrowings of the Company in conjunction with the acquisition;
•A reduction in expenses for the year ended June 30, 2021 and a corresponding increase in the year ended June 30, 2020, for acquisition-related transaction costs directly attributable to the acquisition;
•A reduction in revenues due to the elimination of deferred contract revenue assigned no value at the acquisition date;
•An increase in income tax expense using the U.S. statutory rate of 25% to reflect a change in tax status had the Biodex and SNC results of operations been included in the Company’s consolidated tax return; and
•The related income tax effects of the adjustments noted above.
For the years ended June 30, 2021 and June 30, 2020, pro forma adjustments directly attributable to the acquisitions include: (i) the purchase accounting effect of inventories acquired of $5.2 million, (ii) transaction costs of $4.8 million; and (iii) the reduction in revenues of $14.8 million due to the elimination of deferred contract revenue assigned no value at the acquisition date.
3. Contracts in Progress
Costs and billings on uncompleted construction-type contracts consist of the following (in millions):
| | | | | | | | | | | | | | | | | | | | |
| Successor | | | Predecessor |
| December 31, 2021 | | | June 30, 2021 | | June 30, 2020 |
| Costs incurred on contracts (from inception to completion) | $ | 199.4 | | | | $ | 185.8 | | | $ | 152.5 | |
| Estimated earnings | 125.5 | | | | 133.2 | | | 108.9 | |
| Contracts in progress | 324.9 | | | | 319.0 | | | 261.4 | |
| Less: billings to date | (281.8) | | | | (261.9) | | | (209.8) | |
| Less: write-offs | — | | | | (2.7) | | | — | |
| $ | 43.1 | | | | $ | 54.4 | | | $ | 51.6 | |
The carrying amounts related to uncompleted construction-type contracts are included in the accompanying balance sheets under the following captions (in millions):
| | | | | | | | | | | | | | | | | | | | |
| Successor | | | Predecessor |
| December 31, 2021 | | | June 30, 2021 | | June 30, 2020 |
| Costs and estimated earnings in excess of billings on uncompleted contracts – current | $ | 56.3 | | | | $ | 57.2 | | | $ | 59.5 | |
Costs and estimated earnings in excess of billings on uncompleted contracts – noncurrent (1) | 6.5 | | | | 8.1 | | | — | |
Billings in excess of costs and estimated earnings on uncompleted contracts – current (2) | (17.6) | | | | (8.0) | | | (8.0) | |
Billings in excess of costs and estimated earnings on uncompleted contracts – noncurrent (3) | (2.1) | | | | (2.9) | | | — | |
| $ | 43.1 | | | | $ | 54.4 | | | $ | 51.5 | |
(1)Included in other assets within the consolidated balance sheets.
(2)Included in deferred contract revenue – current within the consolidated balance sheets.
(3)Included in other liabilities within the consolidated balance sheets.
Substantially all of the contract liabilities balance as of June 30, 2020 was recognized as revenue during the year ended June 30, 2021, and substantially all of the contract liabilities balance as of June 30, 2021 was recognized as revenue during the Predecessor Stub Period from July 1, 2021 to October 19, 2021 and the Successor Period from October 20, 2021 to December 31, 2021.
4. Inventories
The components of inventories consist of the following (in millions):
| | | | | | | | | | | | | | | | | | | | |
| Successor | | | Predecessor |
| | December 31, 2021 | | | June 30, 2021 | | June 30, 2020 |
| Raw materials | $ | 56.8 | | | | $ | 50.9 | | | $ | 40.6 | |
| Work in progress | 26.6 | | | | 26.8 | | | 16.1 | |
| Finished goods | 40.2 | | | | 35.5 | | | 33.5 | |
| | $ | 123.6 | | | | $ | 113.2 | | | $ | 90.2 | |
5. Property, Plant and Equipment, Net
Property, plant and equipment, net consist of the following (in millions):
| | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | Successor | | | Predecessor |
| | Depreciable
Lives | | December 31, 2021 | | | June 30, 2021 | | June 30, 2020 |
| Land, buildings, and leasehold improvements | 3-39 years | | $ | 45.0 | | | | $ | 44.4 | | | $ | 43.9 | |
| Machinery and equipment | 5-15 years | | 26.7 | | | | 49.6 | | | 38.9 | |
| Badges | 3-5 years | | 27.9 | | | | 38.9 | | | 29.4 | |
| Furniture, fixtures, computer equipment and other | 3-10 years | | 16.7 | | | | 33.6 | | | 27.6 | |
| Construction in progress | — | | 12.2 | | | | 13.6 | | | 7.3 | |
| | | | 128.5 | | | | 180.1 | | | 147.1 | |
| Less: accumulated depreciation and amortization | | | (4.5) | | | | (91.3) | | | (71.9) | |
| | | | $ | 124.0 | | | | $ | 88.8 | | | $ | 75.2 | |
Total depreciation expense included in costs of revenues and operating expenses was as follows (in millions):
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | Successor | | | Predecessor |
| | December 31, 2021 | | | October 19, 2021 | | June 30, 2021 | | June 30, 2020 | | June 30, 2019 |
| Depreciation expense in: | | | | | | | | | | | |
| Cost of revenues | | $ | 3.5 | | | | $ | 3.9 | | | $ | 14.0 | | | $ | 12.7 | | | $ | 11.1 | |
| Operating expenses | | 1.7 | | | | 2.1 | | | 6.8 | | | 5.2 | | | 5.4 | |
Construction in progress includes capitalized internal use software costs totaling $1.7 million, $3.5 million, and $1.2 million as of December 31, 2021, June 30, 2021, and June 30, 2020 respectively.
6. Accrued Expenses and Other Current Liabilities, and Deferred Income Taxes and Other Long-Term Liabilities
Accrued expenses and other current liabilities consist of the following (in millions):
| | | | | | | | | | | | | | | | | | | | |
| Successor | | | Predecessor |
| | December 31, 2021 | | | June 30, 2021 | | June 30, 2020 |
| Compensation and related benefit costs | $ | 34.0 | | | | $ | 38.9 | | | $ | 30.3 | |
| Customer deposits | 8.8 | | | | 8.1 | | | 3.1 | |
| Accrued commissions | 0.9 | | | | 1.1 | | | 3.7 | |
| Accrued warranty costs | 5.9 | | | | 6.3 | | | 5.5 | |
| Non-income taxes payable | 7.5 | | | | 5.0 | | | 4.9 | |
| Pension and other post-retirement obligations | 0.3 | | | | 0.5 | | | 0.3 | |
| Income taxes payable | 3.2 | | | | 3.1 | | | 9.2 | |
| Restructuring | 1.4 | | | | 3.1 | | | — | |
| Accrued professional fees related to becoming a public company | 1.8 | | | | 8.3 | | | — | |
| Deferred and contingent consideration | 2.0 | | | — | | | — | |
| Other accrued expenses | 9.6 | | | | 9.9 | | | 7.1 | |
| Total | $ | 75.4 | | | | $ | 84.3 | | | $ | 64.1 | |
Deferred income taxes and other long-term liabilities consist of the following (in millions):
| | | | | | | | | | | | | | | | | | | | |
| Successor | | | Predecessor |
| | December 31, 2021 | | | June 30, 2021 | | June 30, 2020 |
| Deferred income taxes | $ | 161.0 | | | | $ | 40.1 | | | $ | 33.1 | |
| Pension and other post-retirement obligations, non-current | 11.7 | | | | 12.5 | | | 12.4 | |
| Other long-term liabilities | 24.7 | | | | 24.9 | | | 18.0 | |
| Total | $ | 197.4 | | | | $ | 77.5 | | | $ | 63.5 | |
7. Goodwill and Intangible Assets
Goodwill
Goodwill is calculated as the excess of consideration transferred over the net assets recognized for acquired businesses and represents future economic benefits arising from the other assets acquired that could not be individually identified and separately recognized. The Company assesses goodwill for impairment at the reporting unit level annually on the first day of the fourth quarter and upon the occurrence of a triggering event or change in circumstance that would more likely than not reduce the fair value of a reporting unit below its carrying amount.
Goodwill is assigned to reporting units at the date the goodwill is initially recorded and is reallocated as necessary based on the composition of reporting units over time. No goodwill impairment was recognized for the Successor Period or
Predecessor Periods from July 1, 2021 through December 31, 2021 and fiscal years ended June 30, 2021, and June 30, 2020.
The following table shows changes in the carrying amount of goodwill by reportable segment as of December 31, 2021 and 2020 (in millions):
| | | | | | | | | | | | | | | | | |
| Predecessor |
| | Medical | | Industrial | | Consolidated |
| Balance—June 30, 2019 | $ | 96.7 | | | $ | 414.9 | | | $ | 511.6 | |
| Acquisition of Capintec | 6.0 | | | — | | | 6.0 | |
| Acquisition of Premium Analyse | — | | | 4.3 | | | 4.3 | |
| Acquisition of Selmic | — | | | 2.7 | | | 2.7 | |
| Acquisition of AWST | 4.1 | | | — | | | 4.1 | |
| Translation adjustment | — | | | (6.1) | | | (6.1) | |
| Balance—June 30, 2020 | $ | 106.8 | | | $ | 415.8 | | | $ | 522.6 | |
| Acquisition of Sun Nuclear | 130.2 | | | — | | | 130.2 | |
| Acquisition of Biodex | 11.1 | | | — | | | 11.1 | |
| Acquisition of Dosimetrics | 1.6 | | | — | | | 1.6 | |
| Translation adjustment | (0.2) | | | 16.2 | | | 16.0 | |
| Balance—June 30, 2021 | $ | 249.5 | | | $ | 432.0 | | | $ | 681.5 | |
| Acquisition of Dosimetry Badge | 0.9 | | | — | | | 0.9 | |
| Balance—Translation adjustment | (0.4) | | | (4.6) | | | (5.0) | |
| Balance—October 19, 2021 | $ | 250.0 | | | $ | 427.4 | | | $ | 677.4 | |
| | | | | | | | | | | | | | | | | |
| Successor |
| Medical | | Industrial | | Consolidated |
| Balance—October 20, 2021 | $ | — | | | $ | — | | | $ | — | |
| Acquisition of Mirion | 675.2 | | | 963.8 | | | 1,639.0 | |
| Acquisition of CHP Badge | 1.5 | | | — | | | 1.5 | |
| Acquisition of Safeline | 0.8 | | | — | | | 0.8 | |
| Acquisition of CIRS | 35.0 | | | — | | | 35.0 | |
| Translation adjustment | — | | | (13.7) | | | (13.7) | |
| Balance—December 31, 2021 | $ | 712.5 | | | $ | 950.1 | | | $ | 1,662.6 | |
A portion of the goodwill is deductible for income tax purposes.
Intangible Assets
Intangible assets consist of our developed technology, customer relationships, backlog, trade names, and non-compete agreements at the time of acquisition through business combinations. The customer relationships definite lived intangible assets are amortized using the double declining balance method while all other definite lived intangible assets are amortized on a straight-line basis over their estimated useful lives.
Many of our intangible assets are not deductible for income tax purposes. A summary of intangible assets useful lives, gross carrying value and related accumulated amortization is below (in millions):
| | | | | | | | | | | | | | | | | | | | | | | |
| Successor |
| | | December 31, 2021 |
| Original Average
Life in Years | | Gross Carrying
Amount | | Accumulated
Amortization | | Net Book
Value |
| Customer relationships | 6-13 | | $ | 341.0 | | | $ | (15.3) | | | $ | 325.8 | |
| Distributor relationships | 7-13 | | 61.0 | | | (1.5) | | 59.5 |
| Developed technology | 5-16 | | 251.2 | | | (5.9) | | 245.3 |
| Trade names | 3-10 | | 100.0 | | | (2.1) | | 97.9 |
| Backlog and other | 1-4 | | 85.7 | | | (7.2) | | 78.4 |
| Total | | | $ | 838.9 | | | $ | (32.0) | | | $ | 806.9 | |
| | | | | | | |
| Predecessor |
| | | June 30, 2021 |
| Original Average
Life in Years | | Gross Carrying
Amount | | Accumulated
Amortization | | Net Book
Value |
| Customer relationships | 6-17 | | $ | 420.4 | | | $ | (205.6) | | | $ | 214.8 | |
| Developed technology | 3-16 | | 184.5 | | | (104.7) | | | 79.8 | |
| Trade names | 5-9 | | 47.4 | | | (29.5) | | | 17.9 | |
| Backlog and other | 1-9 | | 40.6 | | | (26.8) | | | 13.8 | |
| Total | | | $ | 692.9 | | | $ | (366.6) | | | $ | 326.3 | |
| | | | | | | |
| | | June 30, 2020 |
| Original Average
Life in Years | | Gross Carrying
Amount | | Accumulated
Amortization | | Net Book
Value |
| Customer relationships | 6-15 | | $ | 358.5 | | | $ | (170.5) | | | $ | 188.0 | |
| Developed technology | 3-16 | | 130.0 | | | (81.0) | | | 49.0 | |
| Trade names | 5-9 | | 32.9 | | | (22.4) | | | 10.5 | |
| Backlog and other | 1-9 | | 22.8 | | | (22.0) | | | 0.8 | |
| Total | | | $ | 544.2 | | | $ | (295.9) | | | $ | 248.3 | |
Aggregate amortization expense for intangible assets included in cost of revenues and operating expenses was as follows (in millions):
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Successor | | | Predecessor |
|
From October 20, 2021
through December 31, 2021 | | | From July 1, 2021 through
October 19, 2021 | | Fiscal Year Ended June 30, 2021 | | Fiscal Year Ended June 30, 2020 | | Fiscal Year Ended June 30, 2019 |
| Amortization expense for intangible assets in: | | | | | | | | | | |
| Cost of revenues | $ | 5.6 | | | | $ | 6.6 | | | $ | 20.9 | | | $ | 17.9 | | | $ | 18.4 | |
| Operating expenses | $ | 26.4 | | | | $ | 13.1 | | | $ | 41.9 | | | $ | 32.7 | | | $ | 34.5 | |
Future annual amortization expense at current exchange rates is as follows (in millions):
| | | | | |
| Fiscal year ending December 31: | |
| 2022 | $ | 142.9 | |
| 2023 | 126.9 | |
| 2024 | 112.5 | |
| 2025 | 90.4 | |
| 2026 | 83.3 | |
| 2027 and thereafter | 250.9 | |
| Total | $ | 806.9 | |
8. Borrowings
On June 17, 2021, Mirion and other sellers entered into the Business Combination Agreement with GSAH, a special purpose acquisition company. On October 20, 2021, Mirion consummated the Business Combination pursuant to the Business Combination Agreement, combining with a subsidiary of GSAH at the Closing, for total consideration of approximately $2.6 billion. The Sellers received cash consideration of approximately $1.3 billion and 30,401,902 shares of Class A and 8,560,540 shares of Class B common stock valued at approximately $0.4 billion on the Closing Date (based upon $10.45 average- price per share of GSAH's Class A common stock on the Closing Date). The Shareholder Notes and Management Notes were acquired by GSAH at the Closing for a price equal to the full outstanding principal amount together with all accrued but unpaid interest up to but excluding the Closing Date using a portion of the Business Combination Consideration. In connection with the Closing, GSAH contributed the Shareholder Notes and the Management Notes to Mirion TopCo, and then the Shareholder Notes and Management Notes were extinguished in full. Borrowings under the 2019 Credit Facility (as defined below) as of the Closing Date were paid in full through the cash consideration and new financing obtained through the 2021 Credit Agreement described below.
Third-party notes payable consist of the following (in millions):
| | | | | | | | | | | | | | | | | | | | |
| Successor | | | Predecessor |
| December 31,
2021 | | | June 30,
2021 | | June 30,
2020 |
| 2021 Credit Agreement | $ | 828.3 | | | | $ | — | | | $ | — | |
| 2019 Credit Facility – first lien term loan | — | | | | 906.4 | | | 682.1 | |
| NRG Loan | — | | | | — | | | 5.8 | |
| JLG Note Payable | — | | | | 0.3 | | | 0.2 | |
| Canadian Financial Institution | 1.2 | | | | 1.2 | | | 1.2 | |
| Other | 2.3 | | | | 0.8 | | | — | |
| Draw on revolving line of credit | — | | | | — | | | 35.0 | |
| Total third-party borrowings | 831.8 | | | 908.7 | | 724.3 |
| Less: notes payable to third-parties, current | (3.9) | | | | (6.4) | | | (41.1) | |
| Less: deferred financing costs | (21.1) | | | | (16.6) | | | (13.4) | |
| Notes payable to third-parties, non-current | $ | 806.8 | | | | $ | 885.7 | | | $ | 669.8 | |
As of December 31, 2021, the fair market value of the Company's 2021 Credit Agreement was $825.2 million. As of June 30, 2021 and June 30, 2020, the fair market value of the Company’s 2019 Credit Facility – first lien term loan was $906.4 million and $662.5 million, respectively. The fair market value for the 2021 Credit Agreement and 2019 Credit Facility were estimated using primarily level 2 inputs, including borrowing rates available to the Company at the respective period ends. The fair market value for the Company’s remaining third-party debt approximates the respective carrying amounts as of December 31, 2021, June 30, 2021 and June 30, 2020.
2021 Credit Agreement
In connection with the Business Combination, certain subsidiaries of the Company entered into the 2021 Credit Agreement among Mirion Technologies (HoldingSub2), Ltd., a limited liability company incorporated in England and Wales, as Holdings, Mirion Technologies (US Holdings), Inc., as the Parent Borrower, Mirion Technologies (US), Inc., as the Subsidiary Borrower, the lending institutions party thereto, Citibank, N.A., as the Administrative Agent and Collateral Agent and Goldman Sachs Lending Partners, Citigroup Global Markets Inc., Jefferies Finance LLC and JPMorgan Chase Bank, N.A., as the Joint Lead Arrangers and Bookrunners.
The 2021 Credit Agreement refinanced and replaced the credit agreement from March 2019, by and between, among others, Mirion Technologies (HoldingRep), Ltd. ("Mirion HoldingRep"), its subsidiaries and Morgan Stanley Senior Funding Inc., as administrative agent, certain other revolving lenders and a syndicate of institutional lenders (the “2019 Credit Facility”) which is described in more detail below.
The 2021 Credit Agreement provides for an $830.0 million senior secured first lien term loan facility and a $90.0 million senior secured revolving facility (collectively, the “Credit Facilities”). The Credit Facilities are permitted to be used to effect the Transactions (as defined in the 2021 Credit Agreement), refinance the 2019 Credit Facility referred to below and for general corporate purposes. The term loan facility is scheduled to mature on October 20, 2028 and the revolving facility
is scheduled to expire and mature on October 20, 2026. The agreement requires the payment of a commitment fee of 0.50% per annum for unused revolving commitments, subject to stepdowns to 0.375% per annum and 0.25% per annum upon the achievement of specified leverage ratios. Any outstanding letters of credit issued under the 2021 Credit Agreement reduce the availability under the revolving line of credit.
The 2021 Credit Agreement is secured by a first priority lien on the equity interests of the Parent Borrower owned by Holdings and substantially all of the assets (subject to customary exceptions) of the borrowers and the other guarantors thereunder. Interest with respect to the facilities is based on, at the option of the borrowers, (i) a customary base rate formula for borrowings in U.S. dollars or (ii) a floating rate formula based on LIBOR (with customary fallback provisions) for borrowings in U.S. dollars, a floating rate formula based on EURIBOR for borrowings in Euro or a floating rate formula based on SONIA for borrowings in Pounds Sterling, each as described in the 2021 Credit Agreement with respect to the applicable type of borrowing.
The 2021 Credit Agreement contains customary representations and warranties as well as customary affirmative and negative covenants and events of default. The negative covenants include, among others and in each case subject to certain thresholds and exceptions, limitations on incurrence of liens, limitations on incurrence of indebtedness, limitations on making dividends and other distributions, limitations on engaging in asset sales, limitations on making investments, and a financial covenant that the “First Lien Net Leverage Ratio” (as defined in the 2021 Credit Agreement) as of the end of any fiscal quarter is not greater than 7.00 to 1.00 if on the last day of such fiscal quarter certain borrowings outstanding under the revolving credit facility exceed 40% of the total revolving credit commitments at such time. The covenants also contain limitations on the activities of Mirion Technologies (HoldingSub2), Ltd. as the “passive” holding company. If any of the events of default occur and are not cured or waived, any unpaid amounts under the 2021 Credit Agreement may be declared immediately due and payable, the revolving credit commitments may be terminated and remedies against the collateral may be exercised. Mirion Technologies (HoldingSub2), Ltd. and subsidiaries were in compliance with all debt covenants on December 31, 2021.
Term Loan - The term loan has a seven-year term (expiring October 2028), bears interest at the greater of Adjusted London Interbank Offered Rate ("LIBOR") or 0.50%, plus 2.75% and has quarterly principal repayments of 0.25% of the original principal balance. The interest rate was 3.25% at December 31, 2021. The Company repaid $1.7 million for the Successor Period ended December 31, 2021, yielding an outstanding balance of approximately $828.3 million as of December 31, 2021.
Revolving Line of Credit - The revolving line of credit arrangement has a five year term and bears interest at the greater of LIBOR or 0%, plus 2.75%. The agreement requires the payment of a commitment fee of 0.50% per annum for unused commitments. The revolving line of credit matures in October 2026, at which time all outstanding revolving facility loans and accrued and unpaid interest are due. Any outstanding letters of credit reduce the availability of the revolving line of credit. There was no outstanding balance under the arrangement as of December 31, 2021. Additionally, the Company has standby letters of credit issued under its 2021 Credit Agreement that reduce the availability under the revolver of $8.1 million. As of December 31, 2021, the amount available on the revolver was approximately $81.9 million for the same period.
Deferred Financing Costs
In connection with the issuance of the 2021 Credit Agreement term loan, we incurred debt issuance costs of $21.7 million on date of issuance. In accordance with accounting for debt issuance costs, we recognize and present deferred finance costs associated with non-revolving debt and financing obligations as a reduction from the face amount of related indebtedness in our consolidated balance sheet.
In connection with the issuance of the 2021 Credit Agreement revolving line of credit, we incurred debt issuance costs of $1.8 million. We recognize and present debt issuance costs associated with revolving debt arrangements as an asset and include the deferred finance costs within other assets on our consolidated balance sheet. We amortize all debt issuance costs over the life of the related indebtedness.
For the period from the Closing Date through December 31, 2021, we incurred approximately $0.7 million of amortization expense of the deferred finance costs.
2019 Credit Facility
In conjunction with the Business Combination, the 2021 Credit Agreement refinanced and replaced the 2019 Credit Facility.
The 2019 Credit Facility provided for financing of a $450.0 million senior secured term loan facility and a €125.0 million term loan facility, as well as a $90.0 million revolving line of credit. The 2019 Credit Facility was amended to provide an additional $225.0 million, $34.0 million and $66.0 million in gross proceeds from the USD term loan in December 2020, July 2019, and December 2019, respectively.
The 2019 Credit Facility was secured by a first priority lien on substantially all of Mirion HoldingRep and subsidiaries’ assets in the United States, certain assets of guarantor subsidiaries in Germany, the United Kingdom, Canada, France, Belgium and Luxembourg and two-thirds of assets in non-guarantors and other countries. Loan fees recorded as debt discounts are amortized using the effective interest method. The 2019 Credit Facility contained customary restrictive covenants, as well as financial covenants that require Mirion HoldingRep and subsidiaries to maintain a certain total level of debt-to-income ratio and interest coverage ratio, each as defined in the Credit Facility, as well as non-financial affirmative and negative covenants. The negative covenants, subject to certain exceptions, generally limited the ability of Mirion HoldingRep and subsidiaries to incur additional debt, create liens, make fundamental changes, make certain investments, pay dividends, purchase or retire equity interests, or prepay or retire certain debt. Mirion HoldingRep and subsidiaries were in compliance with all debt covenants on June 30, 2021 and through the date of extinguishment.
USD term loan – The term loan had a seven-year term (expiring March 2026), bearing interest at the greater of Adjusted London Interbank Offered Rate (“LIBOR”) or 0%, plus 4.00%, and had quarterly principal repayments of 0.25% of the original principal balance. The interest rate was 4.08%, 4.15% and 5.07% through the Closing Date and as of June 30, 2021 and 2020, respectively. The Company repaid $7.2 million and $5.5 million for the fiscal year ended June 30, 2021 and June 30, 2020, respectively and $1.9 million through the Closing Date, yielding an outstanding balance of approximately $761.3 million and $543.5 million as of June 30, 2021 and June 30, 2020, respectively, and $759.4 million as of the Closing Date.
Euro term loan - The Euro portion of the term loan had a seven-year term (expiring March 2026), bearing interest at the greater of European union interbank market (“Euribor”) or 0%, plus 4.25% and has quarterly principal repayments of 0.25% of the original principal balance. As of June 30, 2021, June 30, 2020 and through the Closing Date, the interest rate was 4.25%. The Company repaid $1.5 million, $1.4 million, $0.4 million for the fiscal year ended June 30, 2021, June 30, 2020 and through the Closing Date, respectively, yielding an outstanding balance of approximately €122.2 million (approximately $145.1 million) and €123.4 million ($138.6 million approximately) as of June 30, 2021 and June 30, 2020, respectively, and €121.9 million (approximately $141.9 million) as of the Closing Date.
Revolving Line of Credit - The revolving line of credit arrangement had a five-year term and bearing interest at the greater of LIBOR or 0%, plus 4.00%. The agreement requires the payment of a commitment fee of 0.50% per annum for unused commitments. The revolving line of credit matures in March 2024, at which time all outstanding revolving facility loans and accrued and unpaid interest are due. Any outstanding letters of credit reduce the availability of the revolving line of credit. There was no outstanding balance under the arrangement as of June 30, 2021. Additionally, the Company has standby letters of credit issued under its Credit Facility that reduce the availability under the revolver of $8.7 million and $9.0 million as of June 30, 2021, and June 30, 2020, respectively, the amount available on the revolver was approximately $81.3 million and $46.0 million, for the same periods, respectively.
Deferred Financing Costs
As noted above, the 2021 Credit Agreement refinanced and replaced the 2019 Credit Facility. In conjunction with the Business Combination purchase accounting we wrote off the remaining unamortized original issue discounts (OID) and debt issuance costs of $15.4 million related to the term loan and $0.4 million related to the revolving line of credit and recorded as a loss on extinguishment of debt on the last day of the Predecessor Period.
In connection with the issuance of the 2019 Credit Facility, we incurred debt issuance costs of $16.3 million on date of issuance, and an additional $6.2 million and $1.2 million of costs for incremental proceeds in fiscal years June 30, 2021 and June 30, 2020, respectively. In conjunction with the issuance of 2019 Credit Facility, we concluded there was an extinguishment of a previous debt. We wrote off the remaining unamortized original issue discounts (OID) and debt issuance costs of $12.8 million in March 2019. In accordance with accounting for debt issuance costs, we recognize and present deferred finance costs associated with non-revolving debt and financing obligations as a reduction from the face amount of related indebtedness in our consolidated balance sheet.
In connection with the issuance of the 2019 Credit Facility revolving line of credit, we incurred debt issuance costs of $0.9 million. We wrote off the remaining unamortized debt issuance costs of $0.2 million of a previous revolving credit agreement in March 2019. We recognize and present debt issuance costs associated with revolving debt arrangements as an
asset and include the deferred finance costs within other assets on our consolidated balance sheet. We amortize all debt issuance costs over the life of the related indebtedness.
During fiscal years ended June 30, 2021, June 30, 2020 and June 30, 2019, we incurred approximately $3.2 million, 2.6 million and $2.8 million, respectively, of amortization expense of the deferred finance costs, in addition to the write off of $12.8 million included in Loss on debt extinguishment in the Predecessor Period fiscal year ended June 30, 2019.
NRG Loan -In conjunction with the acquisition of NRG (see Note 2), the Company entered into a loan agreement for €7.2 million ($7.4 million) at the date of the acquisition. This agreement expires in December 2023. The loan bears interest which is Euribor of three months, plus 2.0%, and mandatory costs if any. This loan was paid off in the fiscal year ended June 30, 2021.
Canadian Financial Institution -In May 2019, the Company entered into a credit agreement for C$1.7M ($1.3 million) with a Canadian financial institution that matures in April 2039. The note bears annual interest at 4.15%. The credit agreement is secured by the facility acquired using the funds obtained.
JLG Note Payable -In May 2019, the Company entered into a note payable for $0.2 million with an individual that has left the organization, which is due upon a change in control of Mirion Technologies (Global), Ltd, a wholly owned subsidiary of the Company. The note bearing annual interest at 6.00% was paid in full as part of the Business Combination.
Overdraft Facilities
The Company has overdraft facilities with certain German and French financial institutions. As of December 31, 2021, June 30, 2021 and June 30, 2020, there were no outstanding amounts under these arrangements.
Accounts Receivable Sales Agreement
We are party to an agreement to sell short-term receivables from certain qualified customer trade accounts to an unaffiliated French financial institution without recourse. Under this agreement, the Company can sell up to €8.0 million ($9.1 million as of December 31, 2021) of eligible accounts receivables. The accounts receivable under this agreement are sold at face value and are excluded from the consolidated balance if revenue has been recognized on the related receivable. When the related revenue has not been recognized on the receivable the Company considers the accounts receivable to be collateral for short-term borrowings. As of December 31, 2021, June 30, 2021 and June 30, 2020, there was approximately $0.4 million, $0.8 million and $0.0 million, respectively, outstanding under these arrangements which were included as Other in the Borrowings table above.
Total costs associated with this arrangement were immaterial for the Successor Periods and for all Predecessor Periods presented and are included in selling, general and administrative expense in the consolidated statement of operations.
Performance Bonds and Other Credit Facilities
The Company has entered into various line of credit arrangements with local banks in France and Germany. These arrangements provide for the issuance of documentary and standby letters of credit of up to €70.3 million ($79.7 million), €67.3 million ($79.9 million), and €47.3 million ($53.1 million) as of December 31, 2021, June 30, 2021 and June 30, 2020, respectively, subject to certain local restrictions. As of December 31, 2021, June 30, 2021, and June 30, 2020 €37.7 million ($42.7 million), €24.7 million ($29.3 million), and €21.2 million ($23.7 million), respectively, of the lines had been utilized to guarantee documentary and standby letters of credit, with interest rates ranging from 0.5% to 2.0%. In addition, the Company posts performance bonds with irrevocable letters of credit to support certain contractual obligations to customers for equipment delivery. These letters of credit are supported by restricted cash accounts, which totaled $1.3 million, $1.0 million and $1.7 million as of December 31, 2021, June 30, 2021 and June 30, 2020 , respectively.
At December 31, 2021, contractual principal payments of total third-party borrowings are as follows (in millions):
| | | | | |
| Fiscal year ending December 31: | |
| 2022 | $ | 7.1 | |
| 2023 | 8.4 | |
| 2024 | 8.3 | |
| 2025 | 8.2 | |
| 2026 | 9.6 | |
| Thereafter | 790.2 | |
| Gross Payments | 831.8 | |
| Unamortized debt issuance costs | (21.1) | |
| Total third-party borrowings, net of debt issuance costs | $ | 810.7 | |
Notes Payable to Related Parties
Concurrent with the Closing, a portion of the Business Combination Consideration was used to extinguish the Shareholder Notes and the Management Notes in full.
Notes payable to related parties consists of the following (in millions):
| | | | | | | | | | | | | | | | | | | | |
| Successor | | | Predecessor |
| December 31,
2021 | | | June 30,
2021 | | June 30,
2020 |
| Shareholder Notes | $ | — | | | | $ | 1,166.8 | | | $ | 983.7 | |
| Management Notes | — | | | | 3.7 | | | 3.4 | |
| Notes payable to related parties | $ | — | | | | $ | 1,170.5 | | | $ | 987.1 | |
The estimated fair value of the Company’s related party debt was approximately $1,170.5 million and $987.1 million as of June 30, 2021 and June 30, 2020, respectively. The fair value of this instrument approximates book value due to the reasonable possibility of redemption prior to the term date.
Shareholder and Management Notes – Mirion Technologies (HoldingSub1), Ltd., was authorized to issue $900.0 million (plus accrued paid in-kind (PIK) interest) of notes to shareholders (“Shareholder Notes”) and up to $5.0 million (plus paid in-kind (PIK) cash and interest) of notes to certain members of management (“Management Notes”). The notes ranked pari passu between each other and other unsecured obligations of the Company. The notes could be prepaid without penalty at the Company’s option and were subordinate in right of payment to any indebtedness of the Company to banks or to other financial institutions (either currently existing or to occur in the future). Certain of the Shareholder and Management Notes were admitted to trading and were on the official listing of The International Stock Exchange (TISE).
During fiscal years ended June 30, 2021 and 2020, an additional $181.5 million and $99.6 million in Shareholder Notes were admitted to trading and are on the official listing of TISE, respectively. From July 1, 2021 through the Closing Date, no additional Shareholder Note or Management Notes were admitted to trading. At June 30, 2021 and 2020, there were $1,158.4 million and $976.9 million in Shareholder Notes issued and outstanding, respectively, as listed on TISE. Of the amount available for trading, $683.9 million relates to principal balance and $474.5 million relates to accrued interest as of June 30, 2021. There was no trading activity related to Shareholder and Management Notes during fiscal year ended June 30, 2021, June 30, 2020 and through the Closing Date. As of June 30, 2021 and June 30, 2020, there were $3.6 million and $3.4 million in Management Notes issued and outstanding, respectively, as listed on TISE.
The notes bore simple annual interest at 11.5% except for $70.0 million of Shareholder Notes added in fiscal year 2021 that bore simple annual interest rate of 6.0% until October 1, 2021 when the interest rate converted to simple annual interest of 11.5%. For the Shareholder Notes, the interest was added to the principal outstanding on December 31 of each year until extinguished and were referred to as Shareholder Funding Bonds on TISE. For the Management Notes, half of the interest was added to the principal outstanding on December 31 of each year until extinguished and was referred to as Management Funding Bonds on TISE, while the remaining half was payable in cash annually. The listing on the TISE for Shareholder and Management Funding Bonds was an optional election and certain shareholders had elected to opt-out of listing their Shareholder Funding Bonds. All other shareholders and management had elected to list their funding bonds on TISE. The
notes were due when the Company completes a public offering, a winding-up, a sale, or on March 30, 2026, whichever occurred first. The redemption price was equal to the outstanding principal plus all accrued and unpaid interest then outstanding.
At June 30, 2021, and June 30, 2020, interest of $64.6 million and $56.3 million was accrued on the Shareholder Notes principal outstanding, respectively, and $0.2 million and $0.1 million was accrued on the Management Notes principal outstanding, respectively. As of December 31, 2020 and December 31, 2019, accrued interest of $113.6 million and $101.3 million, respectively, was added to the principal of the Shareholder Notes; and $0.2 million and $0.2 million, respectively was added to the principal of the Management Notes.
9. Leased Assets
The Company primarily leases certain logistics, office, and manufacturing facilities, as well as vehicles, copiers and other equipment. These operating leases generally have remaining lease terms between 1 month and 30 years, and some include options to extend (generally 1 to 10 years). The exercise of lease renewal options is at the Company’s discretion. The Company evaluates renewal options at lease inception and on an ongoing basis, and includes renewal options that it is reasonably certain to exercise in its expected lease terms when classifying leases and measuring lease liabilities. Lease agreements generally do not require material variable lease payments, residual value guarantees or restrictive covenants. The Company’s financing lease arrangements are not material.
The table below presents the locations of the operating lease assets and liabilities on the consolidated balance sheets as of December 31, 2021 (in millions):
| | | | | | | | | | | |
| | | Successor |
| Balance Sheet Line Item | | December 31, 2021 |
| Operating Lease assets | Operating Lease assets | | $ | 45.7 | |
| Financing Lease assets | Other Assets | | $ | 0.9 | |
| Operating lease liabilities: | | | |
| Current operating lease liabilities | Current operating lease liabilities | | $ | 9.3 | |
| Noncurrent operating lease liabilities | Operating lease liability, non-current | | 40.6 | |
| Total operating lease liabilities: | | | $ | 49.9 | |
| Financing lease liabilities: | | | |
| Current financing lease liabilities | Accrued expenses and other current liabilities | | $ | 0.6 | |
| Noncurrent financing lease liabilities | Deferred income taxes and other long-term liabilities | | 0.3 | |
| Total financing lease liabilities: | | | $ | 0.9 | |
The depreciable lives are limited by the expected lease term for operating lease assets and by shorter of either the expected lease term or economic useful life for financing lease assets.
The Company’s leases generally do not provide an implicit rate, and therefore the Company uses its incremental borrowing rate as the discount rate when measuring the lease liabilities. The incremental borrowing rate represents an estimate of the interest rate the Company would incur at lease commencement to borrow an amount equal to the lease payments on a collateralized basis over the term of the lease within a particular currency environment. The Company used incremental borrowing rates as of July 1, 2021 for leases that commenced prior to that date.
The Company’s weighted average remaining lease term and weighted average discount rate for operating leases as of December 31, 2021 are:
| | | | | | | | |
| | Successor |
| | December 31, 2021 |
| Operating leases | | |
| Weighted average remaining lease term (in years) | | 7.5 |
| Weighted average discount rate | | 4.19 | % |
The table below reconciles the undiscounted future minimum lease payments (displayed by year and in the aggregate) under non-cancelable operating leases with terms of more than one year to the total lease liabilities recognized on the consolidated balance sheets as of December 31, 2021 (in millions):
| | | | | |
| Fiscal year ending December 31: | |
| 2022 | $ | 10.8 | |
| 2023 | 9.4 | |
| 2024 | 8.1 | |
| 2025 | 6.4 | |
| 2026 | 5.0 | |
| 2027 and thereafter | 18.5 | |
| Total undiscounted future minimum lease payments | $ | 58.2 | |
| Less: Imputed interest | (8.3) | |
| Total lease liabilities | $ | 49.9 | |
| |
For the, Successor Period from October 20, 2021 through December 31, 2021 and the Predecessor Stub Period from July 1, 2021 through October 19, 2021 operating lease costs (as defined under ASU 2016-02) were $1.8 million and $3.2 million, respectively. Operating lease costs are included within costs of goods sold, selling, general and administrative, and research and development expenses on the consolidated statements of income and comprehensive income. Short-term lease costs, variable lease costs and sublease income were not material for the periods presented.
Cash paid for amounts included in the measurement of operating lease liabilities was $2.3 million and $3.5 million for the Successor Period from October 20, 2021 through December 31, 2021 and the Predecessor Period from July 1, 2021 through October 20, 2021, respectively, and this amount is included in operating activities in the consolidated statements of cash flows. Operating lease assets obtained in exchange for new operating lease liabilities were $4.1 million and $0.4 million for the Successor Period from October 20, 2021 through December 31, 2021 and Predecessor Period from July 1, 2021 through October 20, 2021, respectively.
10. Commitments and Contingencies
Unconditional Purchase Obligations
The Company has entered into certain long-term unconditional purchase obligations with suppliers. These agreements are non-cancellable and specify terms, including fixed or minimum quantities to be purchased, fixed or variable price provisions, and the approximate timing of payment. Unconditional purchase obligations are as follows (in millions):
| | | | | |
| Fiscal year ending December 31: | |
| 2023 | $ | 12.5 | |
| 2024 | 4.7 |
| 2025 | 2.5 |
| 2026 | 1.1 |
| 2027 | 0.4 |
| 2028 and thereafter | 0.3 |
| Total | $ | 21.5 | |
Litigation
The Company is subject to various legal proceedings, claims, litigation, investigations and contingencies arising out of the ordinary course of business. While the ultimate results of such suits or other proceedings against the Company cannot be predicted with certainty, we believe the resolution of these matters will not have a material effect on our results of operations, financial condition, or cash flows. If we believe the likelihood of an adverse legal outcome is probable and the
amount is reasonably estimable, we accrue a liability in accordance with accounting guidance for contingencies. We consult with legal counsel on matters related to litigation and seek input both within and outside the Company.
On December 30, 2021, Mirion Technologies, Inc. agreed to settle claims for attorneys’ fees related to a demand from a purported stockholder of GSAH related to the Business Combination. The stockholder alleged that a then-proposed amendment to GSAH’s certificate of incorporation failed to provide holders of GSAH’s Class A common stock with a separate class vote with respect to a proposed increase in the number of authorized shares of GSAH’s Class A common stock. Prior to the business combination, GSAH mooted the claim by providing the requested class vote to holders of GSAH’s Class A common stock. As part of the settlement, the Company agreed to pay the stockholder’s counsel $0.7 million, and the stockholder and his counsel provided customary releases to the Company, the former directors of GSAH and certain other persons in connection with any and all claims related to the stockholder’s demand and any alleged entitlement by the stockholder’s counsel to attorneys’ fees or expenses.
11. Income Taxes
The domestic and foreign components of (loss) before provision for income taxes and the provision for income taxes were as follows (in millions):
| | | | | |
| Successor |
| From
October 20, 2021 through
December 31, 2021 |
| United States | $ | (26.8) | |
| Foreign | (3.0) | |
| Net loss before benefit from income taxes | $ | (29.8) | |
| Income tax provision (benefit): | |
| Current: | |
| Federal | $ | — | |
| State and local | 0.8 | |
| Foreign | 3.8 | |
| Total current provision | $ | 4.6 | |
| Deferred: | |
| Federal | $ | (5.4) | |
| State and local | (1.2) | |
| Foreign | (4.8) | |
| Total deferred benefit | $ | (11.4) | |
| Total benefit from income taxes | $ | (6.8) | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | Predecessor |
| | | From
July 1, 2021 through
October 19, 2021 | | Fiscal Year Ended
June 30, 2021 | | Fiscal Year Ended
June 30, 2020 | | Fiscal Year Ended
June 30, 2019 |
| United Kingdom | | | $ | (41.2) | | | $ | (125.3) | | | $ | (118.2) | | | $ | (96.3) | |
| United States | | | (61.2) | | (53.8) | | (24.5) | | (42.0) |
| Other foreign | | | (8.9) | | 14.8 | | 18.1 | | 12.1 |
| Net loss before benefit from income taxes | | | $ | (111.3) | | | $ | (164.3) | | | $ | (124.6) | | | $ | (126.2) | |
| Income tax provision (benefit): | | | | | | | | | |
| Current: | | | | | | | | | |
| United Kingdom | | | 0.1 | | 0.3 | | 0.6 | | 1.1 |
| United States | | | 1.4 | | 2.4 | | (6.2) | | 1.9 |
| Other foreign | | | 2.0 | | 9.4 | | 16.1 | | 9.6 |
| Total current provision | | | $ | 3.5 | | | $ | 12.1 | | | $ | 10.5 | | | $ | 12.6 | |
| Deferred: | | | | | | | | | |
| United Kingdom | | | — | | — | | (0.4) | | (0.3) |
| United States | | | (7.0) | | (15.5) | | 1.3 | | (7.2) |
| Other foreign | | | (2.1) | | (2.5) | | (16.9) | | (9.3) |
| Total deferred benefit | | | $ | (9.1) | | | $ | (18.0) | | | $ | (16.0) | | | $ | (16.8) | |
| Total benefit from income taxes | | | $ | (5.6) | | | $ | (5.9) | | | $ | (5.5) | | | $ | (4.2) | |
The provision (benefit) for income taxes differs from the amount computed by applying the U.S. Federal statutory income tax rate to loss before provision for income taxes as follows:
| | | | | |
| Successor |
| From
October 20, 2021 through
December 31, 2021 |
| Income tax at U.S. Federal statutory rate | 21 | % |
| State and local taxes, net of federal impact | 2 | % |
| Foreign tax rate differential | — | % |
| Change in valuation allowance | 3 | % |
| Stock-based compensation expense | (4) | % |
| Warrant liability change in fair value | 1 | % |
| Other | — | % |
| Total effective income tax rate | 23 | % |
The provision (benefit) for income taxes differs from the amount computed by applying the U.K. statutory income tax rate to loss before provision for income taxes as follows:
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | Predecessor |
| | | From
July 1, 2021 through
October 19, 2021 | | Fiscal Year Ended
June 30, 2021 | | Fiscal Year Ended
June 30, 2020 | | Fiscal Year Ended
June 30, 2019 |
| Income tax at U.K. statutory rate | | | 19 | % | | 19 | % | | 19 | % | | 19 | % |
| Subpart F & GILTI | | | — | % | | (1) | % | | (2) | % | | (4) | % |
| Foreign taxes, including U.S. | | | 1 | % | | (1) | % | | 1 | % | | 3 | % |
| Transaction costs | | | (3) | % | | — | % | | — | % | | — | % |
| Change in valuation allowance | | | (2) | % | | 4 | % | | (8) | % | | 1 | % |
| Unrecognized tax benefits | | | (1) | % | | (1) | % | | 11 | % | | — | % |
| Nondeductible interest expense | | | (7) | % | | (14) | % | | (17) | % | | (14) | % |
| Stock-based compensation expense | | | (2) | % | | — | % | | — | % | | — | % |
| Other | | | — | % | | — | % | | — | % | | (2) | % |
| Total effective income tax rate | | | 5 | % | | 4 | % | | 4 | % | | 3 | % |
Taxes of approximately $28.7 million have not been provided on approximately $220 million of certain earnings and profits and approximately $67.1 million of undistributed GAAP retained earnings of non-U.S. foreign subsidiaries which are permanently reinvested.
The components of the Company’s net deferred tax assets and liabilities consist of the following (in millions):
| | | | | | | | | | | | | | | | | | | | |
| Successor | | | Predecessor |
| December 31, 2021 | | | June 30, 2021 | | June 30, 2020 |
| Deferred tax assets: | | | | | | |
| Net operating loss carryforwards | $ | 24.5 | | | | $ | 29.2 | | | $ | 29.2 | |
| Federal and state credit carryforwards | 13.9 | | | 14.3 | | 16.4 |
| Property, plant and equipment | 0.6 | | | 0.6 | | 2.6 |
| Deferred and other revenue differences | 8.6 | | | 4.0 | | — |
| Interest carryforwards | 12.1 | | | 11.2 | | 4.9 |
| Other reserves and accrued expenses | 15.4 | | | 15.0 | | 9.0 |
| Lease liabilities | 12.5 | | | — | | — |
| Other assets | 2.2 | | | 3.7 | | 4.7 |
| Total deferred tax assets | 89.8 | | | 78.0 | | 66.8 |
| Less: valuation allowance | (20.7) | | | (29.1) | | (29.0) |
| $ | 69.1 | | | | $ | 48.9 | | | $ | 37.8 | |
| | | | | | | | | | | | | | | | | | | | |
| Successor | | | Predecessor |
| December 31, 2021 | | | June 30, 2021 | | June 30, 2020 |
| Deferred tax liabilities: | | | | | | |
| Purchased technologies and other intangibles | $ | (192.1) | | | | $ | (75.0) | | | $ | (58.2) | |
| Deferred and other revenue differences | (7.5) | | | (8.1) | | (0.8) |
| Property, plant and equipment | (11.9) | | | (3.9) | | (3.0) |
| Lease right of use assets | (11.4) | | | — | | — |
| Other liabilities | (1.4) | | | (1.8) | | (4.1) |
| Total deferred tax liabilities | (224.3) | | | (88.8) | | (66.1) |
| Net deferred tax liabilities | $ | (155.2) | | | | $ | (39.9) | | | $ | (28.3) | |
The increase in deferred tax liabilities in the Successor Period is mainly due to the fair valuation of the Company's intangible assets as a result of the Business Combination. See Note 2, Acquisitions. Management regularly evaluates the recoverability of deferred tax assets and recognizes the tax benefit only if reassessment demonstrates that they are more likely than not realizable. At such time, if it is determined that it is more likely than not that the deferred tax assets are realizable, the valuation allowance will be adjusted. In assessing the need for a valuation allowance, management considers all available evidence, both positive and negative, including reversals of existing temporary differences; historical levels of income; expectations and risks associated with estimates of future taxable income; and any ongoing tax planning strategies. At December 31, 2021, the Company evaluated the realizability of the deferred tax assets and concluded that a valuation allowance of $20.7 million mostly relating to U.S. foreign tax credit carryovers and non-U.S. net operating loss carryforwards should continue to be recorded. At June 30, 2021 the valuation allowance was $29.1 million mostly relating to U.S. foreign tax credit carryovers and non-U.S. net operating losses and restricted interest carryforwards. In the Successor Period, the Company reflected the impact of the Business Combination but did not otherwise adjust the valuation allowance. The Company increased the valuation allowance by $1.6 million, $0.1 million, and $10.3 million for the Predecessor Stub Period ended October 19, 2021, and the years ended June 30, 2021, and June 30, 2020, respectively.
| | | | | | | | | | | | | | | | | | | | | | | | | | |
| Successor | | | Predecessor |
| From
October 20, 2021 through
December 31, 2021 | | | From
July 1, 2021 through
October 19, 2021 | | Fiscal Year Ended
June 30, 2021 | | Fiscal Year Ended
June 30, 2020 |
| Valuation allowance balance – beginning of period | $ | 1.0 | | | | $ | 29.1 | | | $ | 29.0 | | | $ | 18.7 | |
| Increases/(decreases) resulting from the Mirion Business Combination | 19.7 | | | — | | | — | | | — | |
| Increases resulting from other business combinations | — | | | | — | | | 0.5 | | 0.3 |
| Other increases | — | | | | 1.6 | | 8.6 | | 10.0 |
| Other decreases | $ | — | | | | $ | — | | | $ | (9.0) | | | $ | — | |
| Valuation allowance balance – end of period | $ | 20.7 | | | | $ | 30.7 | | | $ | 29.1 | | | $ | 29.0 | |
Included in increases/(decreases) resulting from the Mirion Business Combination is a decrease of $(10.0) million primarily related to the change in expected realization of tax attributes in the U.K. A majority of the decrease in the fiscal year ended June 30, 2021 is attributable to a change in the realizability of U.S. deferred tax assets upon the acquisition of Sun Nuclear.
As of December 31, 2021, the Company had U.S. federal, U.S. state, and non-U.S. net operating loss carryforwards of $39.7 million, $58.3 million, and $51.3 million, respectively. A majority of the U.S. federal net operating losses will expire in years ending December 31, 2035 and 2036. A majority of the U.S. state net operating losses will continue to expire in years ending December 31, 2022 through 2041. Materially, the foreign net operating losses have an indefinite carryover period. As of December 31, 2021, the Company had U.S. tax credit carryforwards of $14.0 million available to offset future U.S. federal income taxes payable. U.S. tax credit carryforwards will expire in years ending December 31, 2023 through 2037.
As a result of the Business Combination, Mirion TopCo experienced an ownership change. Management analyzed the impact related to the ability to utilize certain of its non-U.S. net operating loss and tax credit carryforwards after the Business Combination and determined all attributes in the U.K. may be carried forward with no restrictions; however, certain net operating losses in Germany would be forfeited. The Company recorded an impact of approximately $3.8 million in purchase accounting related to the forfeiture of Germany net operating losses. In addition, the Company analyzed the ability to utilize U.S. federal and state tax attribute carryforwards and does not expect the use of these attributes to be limited under Section 382 of the Internal Revenue Code and similar state tax laws. The Company continues to monitor the impact of the ownership change on attributes as future changes in the business could further limit the use of these attributes.
As of December 31, 2021, the Company had $6.6 million of unrecognized tax benefits related to uncertain tax positions, $4.2 million of which would affect its effective tax rate if recognized. Although the timing and outcome of tax settlements are uncertain, it is reasonably possible that during the next twelve months a reduction in unrecognized tax benefits related to the deductibility of certain expenses may occur in the range of zero to $2.8 million due to the expiration of various statutes of limitations and anticipated corrections to the timing of deductions. As of June 30, 2021, the Company had $5.0 million of unrecognized tax benefits related to uncertain tax positions, $3.4 million of which would affect its effective tax rate if recognized. A reconciliation of the beginning and ending amount of unrecognized tax benefits is as follows (in millions):
| | | | | | | | | | | | | | | | | | | | | | | | | | |
| Successor | | | Predecessor |
| From
October 20, 2021 through
December 31, 2021 | | | From
July 1, 2021 through
October 19, 2021 | | Fiscal Year Ended
June 30, 2021 | | Fiscal Year Ended
June 30, 2020 |
| Balance, beginning of period | $ | — | | | | $ | 5.0 | | | $ | 0.8 | | | $ | 13.9 | |
| Increases resulting from the Mirion Business Combination | 6.5 | | | — | | — | | — |
| Current year additions to positions | 0.1 | | | 1.5 | | 2.6 | | — |
| Additions from other business combinations | 0.2 | | | — | | 1.7 | | — |
| Lapse of applicable statute of limitations | — | | | — | | (0.1) | | (13.1) |
| Reductions to prior year positions | (0.2) | | | — | | — | | — |
| Foreign currency translation adjustments | — | | | — | | — | | — |
| Balance, end of period | $ | 6.6 | | | | $ | 6.5 | | | $ | 5.0 | | | $ | 0.8 | |
The Company has recorded $3.1 million, $2.1 million, and $0.8 million of unrecognized tax benefits as non-current income taxes payable as of December 31, 2021, June 30, 2021, and June 30, 2020, respectively. The Company has also recorded $3.5 million, $2.9 million, and zero of unrecognized tax benefits as a reduction of net deferred tax assets included in other liabilities in the accompanying consolidated balance sheets at December 31, 2021, June 30, 2021, and June 30, 2020, respectively.
The Company recognizes interest and penalties related to uncertain tax positions as a component of income tax expense. Accrued interest and penalties as of December 31, 2021, June 30, 2021, and June 30, 2020, were approximately $0.9 million, $0.6 million, and $0.3 million, respectively. The ultimate amount and timing of any future cash settlements cannot be predicted with reasonable certainty.
The Company conducts business globally and, as a result, one or more of its subsidiaries files U.S. federal and state income tax returns and income tax returns in other foreign jurisdictions. In the normal course of business, the Company is subject to examination by taxing authorities throughout the world, including such major jurisdictions as the United Kingdom, France, Germany, Canada, and the United States. With the exception of the 2015 tax year returns for Canada, and a few insignificant jurisdictions, the Company is no longer subject to U.S. federal or non-U.S. income tax examinations for years prior to June 30, 2016. The Company is no longer subject to U.S. state and local income tax examinations for years prior to the 2004 tax year.
In many cases, the Company’s uncertain tax positions are related to tax years that remain subject to examination by tax authorities. The following describes open tax years by major tax jurisdictions as of December 31, 2021:
| | | | | |
| | Years Open |
| Jurisdiction: | |
| Canada | 2015 – 2021 |
| France | 2019 – 2021 |
| Germany | 2016 – 2021 |
| United Kingdom | 2016 – 2021 |
| United States—Federal | 2016 – 2021 |
| United States—State | 2004 – 2021 |
12. Supplemental Disclosures to Consolidated Statements of Cash Flows
Supplemental cash flow information and schedules of non-cash investing and financing activities (in millions):
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Successor | | | Predecessor |
|
From
October 20, 2021
through
December 31, 2021 | | | From July 1, 2021 through
October 19, 2021 | | Fiscal Year Ended
June 30, 2021 | | Fiscal Year Ended
June 30, 2020 | | Fiscal Year Ended
June 30, 2019 |
| Cash Paid For: | | | | | | | | | | |
| Cash paid for interest | $ | 5.5 | | | | $ | 10.0 | | | $ | 37.4 | | | $ | 39.2 | | | $ | 39.2 | |
| Cash paid for income taxes | $ | 2.9 | | | | $ | 4.3 | | | $ | 19.3 | | | $ | 10.6 | | | $ | 11.3 | |
| Non-Cash Investing and Financing Activities: | | | | | | | | | | |
| Contingent consideration from acquisitions | $ | 0.5 | | | | $ | 0.8 | | | $ | — | | | $ | — | | | $ | — | |
| Property, plant, and equipment purchases in accounts payable | $ | 0.1 | | | | $ | (1.8) | | | $ | 3.2 | | | $ | 2.0 | | | $ | 2.7 | |
| Acquisition purchases in accrued expense and other liabilities | $ | — | | | | $ | 0.1 | | | $ | 2.1 | | | $ | 2.8 | | | $ | — | |
| Accounts payable converted to note payable to third parties | $ | — | | | | $ | — | | | $ | — | | | $ | — | | | $ | 0.2 | |
| Common Shares issued to Mirion Sellers in Mirion Business Combination | $ | 420.7 | | | | $ | — | | | $ | — | | | — | | | — | |
The following table provides a reconciliation of cash, cash equivalents and restricted cash reported within the consolidated balances sheets that sum to the total of the same such amounts shown in the consolidated statements of cash flow (in millions).
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Successor | | | Predecessor |
|
From
October 20, 2021
through
December 31, 2021 | | | From July 1, 2021 through
October 19, 2021 | | Fiscal Year Ended
June 30, 2021 | | Fiscal Year Ended
June 30, 2020 | | Fiscal Year Ended
June 30, 2019 |
| Cash and cash equivalents | $ | 84.0 | | | | $ | 101.8 | | | $ | 101.1 | | | $ | 118.4 | | | $ | 35.8 | |
| Restricted cash—current | 0.6 | | | | 0.8 | | | 0.8 | | | 1.1 | | | 1.4 | |
| Restricted cash—non-current | 0.7 | | | | 0.5 | | | 0.5 | | | 0.5 | | | 0.4 | |
| Total cash, cash equivalents, and restricted cash shown in the statements of cash flow | $ | 85.3 | | | | $ | 103.1 | | | $ | 102.4 | | | $ | 120.0 | | | $ | 37.6 | |
Amounts included in restricted cash represent funds with various financial institutions to support performance bonds with irrevocable letters of credit for contractual obligations to certain customers.
13. Employee Benefit Plans
Defined Benefit Pension Plans
The Company maintains contributory and noncontributory defined benefit plans for certain employees in France, Japan and Germany. Plan benefits are generally based on each employee’s years of service and final salary. The unfunded benefit obligation recognized in the consolidated balance sheets related to these plans was $11.4 million, $12.3 million, and $11.9 million at December 31, 2021, June 30, 2021, and June 30, 2020, respectively. Benefits expense related to these plans was $0.3 million, $0.3 million, $1.2 million, $1.2 million, and $1.1 million for the Successor Period from October 20, 2021 through December 31, 2021, the Predecessor Periods from July 1, 2021 through October 19, 2021, and the fiscal years ending June 30, 2021, June 30, 2020 and June 30, 2019, respectively. The amount recognized in accumulated other comprehensive loss related to these plans was $1.6 million, $2.2 million, and $2.8 million at December 31, 2021, June 30,
2021, and June 30, 2020, respectively. The estimated future benefit payments over the next ten years are $5.1 million. The estimated gains and losses, net, that will be amortized from accumulated other comprehensive income into benefits expense over the next fiscal year are not significant.
Other Post-Retirement Benefit Plans
The Company maintains a post-retirement benefit plan for certain eligible employees in the United States. Under the provisions of the plan, certain retired employees will secure their own health insurance coverage, and the Company will reimburse the retired employee an amount specified in the plan. The unfunded benefit obligation recognized in the consolidated balance sheets related to this plan was $0.6 million, $0.7 million and $0.7 million at December 31, 2021, June 30, 2021, and June 30, 2020, respectively. Benefits expense related to these plans was negligible for all periods presented. The Company also offers a discretionary retirement plan to certain eligible employees whereby they may defer a portion of their compensation until retirement.
Defined Contribution Plans
The Company maintains 401(k) savings plans and other voluntary defined contribution retirement plans for other eligible employees. Under each plan, eligible employees may make voluntary contributions, while the Company makes contributions as defined by each plan agreement. Employee contributions in each plan are fully vested and Company contributions vest based on years of service in accordance with the provisions of each plan agreement. Total benefits expense for all defined contribution retirement plans was $0.6 million, $1.0 million, $3.7 million, $3.1 million, and $1.7 million for the Successor Period from October 20, 2021 through December 31, 2021, Predecessor Period from July 1, 2021 through October 19, 2021, and the fiscal years ended June 30, 2021, 2020 and 2019, respectively.
14. Stock-Based Compensation
Stock-based compensation is rewarded to employees and directors of the Company and accounted for in accordance with ASC 718, "Compensation—Stock Compensation". Stock-based compensation expense is recognized for equity awards over the vesting period based on their grant-date fair value. Stock-based compensation expense is included within the same financial statement caption where the recipient’s other compensation is reported. The Company accounts for forfeitures as they occur. The Company uses various forms of long-term incentives including, but not limited to RSUs and PSUs, provided that the granting of such equity awards was contingent upon the Company filing Form S-8 with the SEC, which occurred on December 27, 2021.
In conjunction with entering to the Business Combination Agreement, on June 17, 2021 the Sponsor issued the Profits Interests to certain Mirion employees and the current Chairman of the Board of Mirion which are described in more detail below. These awards are treated as stock-based compensation under ASC Topic 718. As the Profits Interests included the completion of the Business Combination as a vesting condition, the expense that accumulated prior to the Business Combination was recorded on the last day of the Predecessor Period.
2021 Omnibus Incentive Plan
We adopted and obtained stockholder approval at the special meeting of the stockholders on October 19, 2021 of the 2021 Plan.
We have reserved 19,952,329 shares of our Class A common stock for issuance pursuant to awards under the 2021 Plan. The total number of shares of our Class A common stock available for issuance under the 2021 Plan will be increased on the first day of each fiscal year following the date on which the 2021 Plan was adopted in an amount equal to the least of (i) three percent (3%) of the outstanding shares of Class A common stock on the last day of the immediately preceding fiscal year, (ii) 9,976,164 shares of Class A common stock and (iii) such number of shares of Class A common stock as determined by the Committee (as defined and designated under the 2021 Plan) in its discretion. Any employee, director or consultant of the Company or any of its subsidiaries or affiliates is eligible to receive an award under the 2021 Plan, to the extent that an offer of such award is permitted by applicable law, stock market or exchange rules, and regulations or accounting or tax rules and regulations.
The 2021 Plan provides for the grant of stock options (including incentive stock options and non-qualified stock options), stock appreciation rights, restricted stock, RSUs, PSUs, other share-based awards, or any combination thereof. Each award will be set forth in a separate grant notice or agreement and will indicate the type and terms and conditions of the award.
The purpose of the 2021 Plan is to motivate and reward employees and other individuals to perform at their highest level and contribute significantly to the success of the Company.
As of December 31, 2021, the pool of shares in the 2021 Plan is summarized as follows:
| | | | | |
| Maximum allowed for issuance | 19,952,329 | |
| Awards granted | 1,238,683 | |
| Awards forfeited | — | |
| Available for future awards | 18,713,646 | |
| Awards vested | — | |
The table below summarizes certain data for our stock-based compensation plans (in millions):
| | | | | | | | | | | |
| Successor | | Predecessor |
| From
October 20, 2021
through
December 31, 2021 | | From July 1, 2021 through
October 19, 2021 |
Stock-based compensation expense (1) | $ | 5.3 | | | $ | 9.3 | |
Tax (expense) benefit for stock-based compensation (2) | $ | — | | | $ | — | |
(1) Includes expense related to Profits Interests for the periods presented. Stock based compensation expense related to RSUs, PSUs and
Director RSUs was immaterial for the periods presented
(2) Tax (expense) benefit was zero related to Profits Interests expense
Restricted Stock Units
RSUs represent a right to receive one share of our Class A common stock that is both nontransferable and forfeitable unless and until certain conditions are satisfied. Certain RSUs vest ratably over various service periods ranging from three to four years. The fair value of the RSUs is determined using the Company’s share price on the grant date.
Performance-based Restricted Stock Units
PSUs vest over a three year performance period. The number of PSUs to be earned is determined based upon attainment of specific business performance goals over the course of the performance period. If certain minimum performance levels are not attained in the performance period, none of the PSUs will become vested. PSUs are considered variable in that compensation could range from zero to 100% of the award agreement's target contingent on the performance level attained. The fair value of the PSUs is determined using a Monte Carlo simulation model determined on the grant date with the following assumptions:
| | | | | |
| MIR Stock Price | $ | 10.70 | |
Expected volatility(1) | 41.12 | % |
Risk-free interest rate(2) | 0.98 | % |
| Dividend yield | 0.00 | % |
| Fair value | $ | 7.91 | |
(1) Expected volatility is based on historical volatilities from a group of comparable entities for a time period similar to
that of the expected term.
(2) The risk-free rate is based on an average of U.S. Treasury yields in effect at the time of grant corresponding with the
expected term.
Director Restricted Stock Units
Members of the Company's Board of Directors ("Director(s)") may elect to receive their quarterly retainer fees in the form of Class A common shares that are covered under our effective Registration Statement on Form S-8. The retainers are paid quarterly, in arrears in the form of cash or stock at the Director's election. Directors also receive annual grants of RSUs
("Director RSUs") that vest quarterly in four installments over the four quarters of the Director's service following the grant date. The number of RSUs granted is determined by the closing price of Mirion's Class A common stock on the grant date.
Activity of our RSUs, PSUs and Director RSUs is as follows:
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| RSUs | | PSUs | | Director RSUs |
| Quantity | | Weighted average grant date fair value | | Quantity | | Weighted average grant date fair value | | Quantity | | Weighted average grant date fair value |
| Beginning balance at Business Combination | — | | | $ | — | | | — | | | $ | — | | | — | | | $ | — | |
| Awards granted | 974,775 | | | $ | 10.48 | | | 229,006 | | | $ | 9.20 | | | 34,902 | | | $ | 10.48 | |
| Awards vested | — | | | $ | — | | | — | | | $ | — | | | — | | | $ | — | |
| Awards forfeited | — | | | $ | — | | | — | | | $ | — | | | — | | | $ | — | |
| Total awards outstanding at December 31, 2021 | 974,775 | | | $ | 10.48 | | | 229,006 | | | $ | 9.20 | | | 34,902 | | | $ | 10.48 | |
Unrecognized compensation cost and weighted average periods remaining for non-vested awards as of December 31, 2021 are as follows (in millions):
| | | | | | | | |
| Successor | |
| From October 20, 2021 through December 31, 2021 | Weighted average period remaining for non-vested awards as of December 31, 2021 |
| Unrecognized compensation cost | | |
| RSUs | $ | 10.2 | | 4 years |
| PSUs | 2.1 | 3 years |
| Director RSUs | 0.4 | 4 months |
| Total unrecognized compensation cost at December 31, 2021 | $ | 12.7 | | |
Profits Interests
In conjunction with entering into the Business Combination Agreement, on June 17, 2021 the Sponsor issued 3,200,000 Profits Interests to Thomas Logan, the Chief Executive Officer of Mirion, 700,000 Profits Interests to Brian Schopfer, the Chief Financial Officer of Mirion, and 4,200,000 Profits Interests to Lawrence Kingsley, the current Chairman of the Board of Mirion. The Profits Interests are intended to be treated as profits interests for U.S. income tax purposes, pursuant to which Messrs. Logan, Schopfer and Kingsley will have an indirect interest in the founder shares held by the Sponsor.
The Profits Interests are subject to service vesting conditions (fifty percent (50%) of the Profits Interests granted to each of Messrs. Logan and Schopfer service-vest on each of the second and third anniversaries of the Closing, and fifty percent (50%) of the Profits Interests granted to Mr. Kingsley service-vest on each of the first and second anniversaries of the Closing) and performance vesting conditions (under which the price per share of Mirion's Class A common stock price must meet or exceed certain established thresholds for 20 out of 30 trading days before the fifth anniversary of the Closing Date). The expense will be recognized on a straight-line basis over the related service period for each tranche of awards.
As the Profits Interests included the completion of the Business Combination as a vesting condition, the expense that accumulated prior to the Business Combination was recorded on the last day of the Predecessor Period.
Of the Profits Interests, $3.2 million have a performance vesting threshold price of $12 per share of Mirion Class A common stock, $2.0 million have a threshold price of $14 per share of Mirion Class A common stock, and $3.0 million have a threshold price of $16 per share of Mirion Class A common stock. Based upon a valuation model using Monte Carlo simulations, a fair value price per share of $8.03, $6.83, and $5.74 has been estimated for the $12, $14, and $16 price per share performance vesting conditions, respectively. The fair value of the Profits Interests are estimated based on a valuation model using Monte Carlo simulations, for the $12, $14, and $16 per share performance vesting conditions, with the following assumptions:
| | | | | |
Cost of equity (1) | 8.5 | % |
Risk-free interest rate (2) | 0.1 | % |
Expected volatility (3) | 30.0 | % |
Expected term (in years) (4) | 5 |
| Average fair value of all profits interests | $ | 6.90 | |
(1) Cost of equity based on a group of comparable entities
(2) The risk-free rate is based on an average of U.S. Treasury yields in effect at the time of grant corresponding with the expected term.
(3) Expected volatility is based on historical volatilities from a group of comparable entities for a time period similar to
that of the expected term and the expected term.
(4) Expected term is based on probability and expected timing of market events.
No additional Profits Interests have been granted after the June 17, 2021 grant. As of December 31, 2021 there is $41.3 million of unrecognized expense to be recognized over a weighted average period of approximately 2 years.
Predecessor Period
Prior to the Business Combination, the Company accounted for share-based compensation related to restricted share awards granted to certain employees by recognizing the grant date fair value of the awards over the requisite service period, which is equal to the vesting period. The Company had the option to buy back the unvested awards upon termination of employment at the lesser of the original issuance price paid by employees or the fair value of the shares on the buy-back date. The Company estimated the value of the restricted share awards by using the Black-Scholes option valuation model, which requires the use of certain subjective assumptions. Significant assumptions include management’s estimates of the estimated share price volatility, the expected life of the awards and related employee forfeiture rates.
As of the Closing Date, Mirion TopCo's board of directors had authorized the issuance of 1,483,795 A Ordinary shares for the fair value at the time of issuance (the "Predecessor Shares"). The Predecessor Shares were issued subject to certain vesting conditions, restrictions on transfer and repurchase rights by Mirion, other employees of the Company or by investors in Mirion. Under the service-vesting conditions, the Predecessor Shares, vested over four years, with one-quarter vesting after one year of service, and the remainder vesting in equal installments over the subsequent thirty-six months.
Vesting of all Predecessor Shares was subject to acceleration in the event of certain change of control transactions. The Predecessor Shares had voting rights and participated in dividends and distributions, if declared; however, the holders of the Predecessor Shares forfeited their voting rights upon termination of employment regardless of vesting status.
The unvested Predecessor Shares were subject to repurchase at a price equal to the lesser of (i) the fair value at the issuance date or (ii) the fair value of the Predecessor Shares as determined on the repurchase date. The Company determined that this repurchase right was a forfeiture provision and accounted for the Predecessor Shares issued to management as a share-based compensation arrangement, with a requisite service period of 4 years. The fair value of the Predecessor Shares was estimated using the Black-Scholes option-pricing model, with the following assumptions:
| | | | | | | | | | | | | | | | | |
| | | Predecessor |
| | | June 30,
2020 | | June 30,
2019 |
| Dividend yield | | | 0.0 | % | | 0.0 | % |
Risk-free interest rate (1) | | | 0.2 | % | | 2.7 | % |
Expected volatility (2) | | | 55.7 | % | | 25.1 | % |
Expected term (in years) (3) | | | 3 | | 2 |
| Fair value | | | $ | 0.37 | | | $ | 0.16 | |
(1) The risk-free rate is based on an average of U.S. Treasury yields in effect at the time of grant corresponding with the expected term.
(2) Expected volatility is based on historical volatilities from a group of comparable entities for a time period similar to
that of the expected term and the expected term.
(3) Expected term is based on probability and expected timing of market events.
No Predecessor Shares were issued during the fiscal year ended June 30, 2021 or during the Predecessor Stub Period from July 1, 2021 through October 19, 2021.
A summary of restricted stock activity within the Company’s equity plans and changes for the years ended June 30, 2021, 2020 and 2019 and the Predecessor Stub Period, is as follows:
| | | | | | | | | | | | | | | | | |
| | Shares (in millions) | | Weighted Average Grant- Date Fair Value | | Total Fair Value (in millions) |
| Restricted Stock Awards | | | | | |
Nonvested at June 30, 2019 | 0.4 | | | $ | 0.39 | | | $ | 0.2 | |
| Granted | 0.2 | | | 0.37 | | | 0.1 | |
| Vested | (0.1) | | | 0.27 | | | — | |
| Repurchased | (0.1) | | | 0.57 | | | (0.1) | |
Nonvested at June 30, 2020 | 0.4 | | | $ | 0.41 | | | $ | 0.1 | |
| Granted | — | | | — | | | — | |
| Vested | (0.2) | | | 0.35 | | | (0.1) | |
| Repurchased | — | | | — | | | — | |
Nonvested at June 30, 2021 | 0.2 | | | $ | 0.27 | | | $ | — | |
| Granted | — | | | — | | | — | |
| Vested | (0.2) | | | 0.27 | | | — | |
| Repurchased | — | | | — | | | — | |
Nonvested at October 19, 2021 | — | | | $ | — | | | $ | — | |
The Company repurchased from and reissued 144,219 Predecessor Shares to members of the management team during fiscal year ended June 30, 2021. Any forfeited Predecessor Shares of restricted common stock were treated as a cancellation with remaining unrecognized expense for the unvested awards recognized on the date of cancellation. The Company did not reverse previously recognized compensation expenses as a result of these cancellations. No Predecessor Shares were repurchased or reissued during the fiscal year ended June 30, 2021 and through October 19, 2021.
Total share-based compensation expense in our consolidated statement of operations and comprehensive loss for fiscal years June 30, 2021, 2020 and 2019 was $0.0 million, $0.2 million, and $0.1 million, respectively. The total value of the Predecessor Shares is amortized as compensation expense ratably over the vesting period of each individual tranche, beginning at the grant date.
15. Related-Party Transactions
Founder Shares
As of the closing of the Business Combination, the Sponsor owned 18,750,000 shares of Class B common stock the ("Founder Shares") which automatically converted into 18,750,000 shares of Class A common stock at the closing of the Business Company. The Founder Shares, are subject to certain vesting and forfeiture conditions and transfer restrictions, as described in more detail below.
The Sponsor and its officers and directors have agreed not to transfer, assign or sell any Founder Shares held by them until the earlier to occur of: (i) October 20, 2022, (ii) the day following the trading day when the last sale price of Class A common stock equals or exceeds $12.00 per share (as adjusted for stock splits, stock dividends, reorganizations, recapitalizations and the like) for any 20 trading days within any 30-trading day period commencing at least 150 days after the Business Combination, and (iii) the date the Company completes a liquidation, merger, stock exchange, reorganization or other similar transaction that results in all of the public stockholders having the right to exchange their shares of common stock for cash, securities or other property. The Sponsor also has certain registration rights with respect to the Founder Shares described below.
Pursuant to the Second Amended and Restated Sponsor Agreement, dated as of October 20, 2021, the Founder Shares also
became subject to vesting in three equal tranches, based on the volume-weighted average price of the Class A common
stock being greater than or equal to $12.00, $14.00 and $16.00 per share for any 20 trading days in any 30 consecutive trading day period. Vesting of the Founder Shares will be accelerated upon certain sale events based on the per share price of the Company’s Class A common stock in such sale event. Holders of the Founder Shares are entitled to vote such
Founder Shares and receive dividends and other distributions with respect to such Founder Shares prior to vesting, but
such dividends and other distributions with respect to unvested Founder Shares will be set aside by the Company and shall
only be paid to the holders of the Founder Shares upon the vesting of such Founder Shares. The Founder Shares will be
forfeited to the Company for no consideration if they fail to vest before October 20, 2026.
Private Placement Warrants
The Sponsor purchased an aggregate of 8,500,000 private placement warrants (the "Private Placement Warrants") at a price of $2.00 per whole warrant ($17 million in the aggregate) in a private placement (the “Private Placement”) that closed concurrently with the closing of GSAH's initial public offering (the "IPO"). Each Private Placement Warrant is exercisable for one whole share of Class A common stock at a price of $11.50 per share, subject to adjustment in certain circumstances, including upon the occurrence of certain reorganization events. The Private Placement Warrants are non-redeemable and exercisable on a cashless basis so long as they are held by the Sponsor or its permitted transferees.
The Private Placement Warrants are accounted for as liabilities as they contain terms and features that do not qualify for equity classification under ASC 815. The fair value of the Private Placement Warrants at December 31, 2021 was $21.2 million.
The Sponsor and GSAH’s officers and directors have agreed, subject to limited exceptions, not to transfer, assign or sell any of their Private Placement Warrants until 30 days after the completion of the Business Combination.
Profits Interests
In connection with the Business Combination Agreement, the Sponsor issued 8,100,000 Profits Interests to certain individuals affiliated with or expected to be affiliated with Mirion after the Business Combination. The holders of the Profits Interests will have an indirect interest in the Founder Shares held by the Sponsor. The Profits Interests are subject to service and performance vesting conditions, including the occurrence of the Closing, and do not fully vest until all of the applicable conditions are satisfied. In addition, the Profits Interests are subject to certain forfeiture conditions. See Note 14, Stock-based Compensation, for further detail regarding the Profits Interests.
Registration Rights
The holders of the Founder Shares and Private Placement Warrants are entitled to registration rights to require the Company to register the resale of any the Founder Shares and the shares underlying the Private Placement Warrants upon exercise pursuant to the Amended and Restated Registration Rights Agreement dated October 20, 2021 (the "RRA'). These holders are also entitled to certain piggyback registration rights. The RRA also includes customary indemnification and confidentiality provisions. The Company will bear the expenses incurred in connection with the filing of any registration statements filed pursuant to the terms of the RRA, including those expenses incurred in connection with the shelf-registration statement on Form S-1 filed on October 27, 2021 and declared effective on November 2, 2021.
Subscription Agreements
Concurrently with the execution of the Business Combination Agreement, the Company entered into a Subscription Agreement with GSAM Holdings LLC, pursuant to, and on the terms and subject to the conditions of which, GSAM Holdings LLC subscribed for 20,000,000 PIPE Shares of the Company’s Class A common stock for an aggregate purchase price equal to $200 million, subject to GSAM Holdings LLC’s rights to syndicate prior to the Closing. The PIPE Investment, including the syndication, was consummated substantially concurrently with Closing.
Related Party Sponsor Note
On November 12, 2020, the Sponsor agreed to loan the Company up to an aggregate of $2 million pursuant to the working capital note (the “Working Capital Note”). Any amounts borrowed under the Working Capital Note were non-interest bearing, unsecured and due at the closing of the Business Combination. The Working Capital Note of $2 million was forgiven in the Successor Period as reflected on the Consolidated Statement of Stockholders Equity (Deficit).
Underwriting Commission
The Company paid an underwriting commission of 2.0% of the gross proceeds of the GSAH's IPO (or $15 million) to the underwriters at the closing of the IPO, of which $11.3 million was paid to an affiliate of the Sponsor. In addition, deferred underwriting discounts and commissions were paid to the underwriters, at the completion of the Business Combination. The deferred underwriting discounts and commissions of $26.3 million were recorded as a current liability on the balance sheet as of June 30, 2021 by GSAH, of which $19.7 million was payable to an affiliate of the Sponsor.
Charterhouse Capital Partners LLP
The Company had entered into agreements with its Predecessor Period primary investor, Charterhouse Capital Partners LLP ("CCP"), which obligated the Company to pay quarterly management fees of $0.1 million per year. In return, CCP provided various investment banking services relating to financing arrangements, mergers and acquisitions and other services. During the Predecessor Stub Period ended October 19, 2021, the Company paid CCP $0.1 million and during the fiscal years ended June 30, 2021, and June 30, 2020 and June 30, 2019 the Company paid CCP an aggregate of $0.1 million, and $0.3 million, and $0.2 million, respectively, for professional fees and expense reimbursements. Upon the completion of the Business Combination, the agreement with CCP is complete. Therefore, as of December 31, 2021 the Company had no additional payments for professional fees or expense reimbursements.
Receivable from Employees for Purchase of Ordinary Shares
As discussed in Note 14, Stock-based Compensation, the Company had made loans to certain members of the management team, to acquire the ordinary shares at fair value, which were paid back to the Company over the requisite service period. As of June 30, 2021 and June 30, 2020 the outstanding balance approximated $2.4 million and $2.7 million, respectively as classified within stockholders’ equity on the Company’s consolidated balance sheet as it represents a receivable in payment of shares. Payments made by the related employees were recorded as an increase to stockholders’ equity. Upon completion of the Business Combination, the loans were paid off or extinguished and therefore as of December 31, 2021 the Company had no outstanding balance.
16. Segment Information
The Company manages its operations through two operating and reportable segments: Medical and Industrial. These segments align the Company’s products and service offerings with customer use in medical and industrial markets and are consistent with how the Company’s Chief Executive Officer, its Chief Operating Decision Maker (“CODM”), reviews and evaluates the Company’s operations. The CODM allocates resources and evaluates the financial performance of each operating segment. The Company’s segments are strategic businesses that are managed separately because each one develops, manufactures and markets distinct products and services. Prior period information herein has been conformed to the current reportable segment structure.
Description of Segments
The Medical segment provides radiation oncology quality assurance, delivering patient safety solutions for diagnostic imaging and radiation therapy centers around the world, dosimetry solutions for monitoring the total amount of radiation medical staff members are exposed to over time, radiation therapy quality assurance solutions for calibrating and verifying imaging and treatment accuracy, and radionuclide therapy products for nuclear medicine applications such as shielding, product handling, medical imaging furniture, and rehabilitation products.
The Industrial segment provides robust, field ready personal radiation detection and identification equipment for defense applications and radiation detection and analysis tools for power plants, labs, and research applications. Nuclear power plant product offerings are used for the full nuclear power plant lifecycle including core detectors and essential measurement devices for new build, maintenance, decontamination and decommission equipment for monitoring and control during fuel dismantling and remote environmental monitoring.
The following table summarizes select operating results for each reportable segment. The CODM evaluates operating results and allocates capital resources among segments, in part, based on segment income from operations, which includes revenues of the segment less expenses that are directly related to those revenues, including purchase accounting impacts to revenue and cost of revenues, but excluding certain charges to cost of revenues and selling, general and administrative expenses predominantly related to corporate costs, shared overhead and other non-operational costs related to restructuring activities and costs to achieve operational initiatives, which are included in "Corporate and Other" in the table below. Interest expense, loss on debt extinguishment, foreign currency loss (gain), net, and other expense (income), net, are not allocated to segments.
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Successor | | | Predecessor |
| (in millions) | From
October 20, 2021
through
December 31, 2021 | | | From July 1, 2021 through
October 19, 2021 | | For Year Ended June 30. 2021 | | For Year Ended June 30. 2020 | | For Year Ended June 30, 2019 |
| Revenues | | | | | | | | | | |
| Medical | $ | 49.2 | | | | $ | 60.3 | | | $ | 155.7 | | | $ | 62.6 | | | $ | 42.9 | |
| Industrial | 104.9 | | | | 107.7 | | | 455.9 | | | 415.6 | | | 397.2 | |
| Consolidated Revenues | $ | 154.1 | | | | $ | 168.0 | | | $ | 611.6 | | | $ | 478.2 | | | $ | 440.1 | |
| Segment Income from Operations | | | | | | | | | | |
| Medical | $ | (4.3) | | | | $ | 0.7 | | | $ | 6.0 | | | $ | 13.9 | | | $ | 10.2 | |
| Industrial | 1.1 | | | | 11.7 | | | 81.5 | | | 59.6 | | | 55.0 | |
| Total Segment Income from Operations | (3.2) | | | | 12.4 | | | 87.5 | | | 73.5 | | | 65.2 | |
| Corporate and other | (19.7) | | | | (54.0) | | | (76.3) | | | (50.5) | | | (36.4) | |
| Consolidated Income from Operations | $ | (22.9) | | | | $ | (41.6) | | | $ | 11.2 | | | $ | 23.0 | | | $ | 28.8 | |
| Capital Expenditures | | | | | | | | | | |
| Medical | $ | 3.8 | | | | $ | 6.8 | | | $ | 14.2 | | | $ | 10.1 | | | $ | 8.0 | |
| Industrial | 2.0 | | | | 2.7 | | | 12.2 | | | 11.4 | | | 10.4 | |
| Total operating and reportable segments | 5.8 | | | | 9.5 | | | 26.4 | | | 21.5 | | | 18.4 | |
| Corporate and other | 0.3 | | | | 0.3 | | | — | | | 0.4 | | | 0.8 | |
| Total Capital Expenditures | $ | 6.1 | | | | $ | 9.8 | | | $ | 26.4 | | | $ | 21.9 | | | $ | 19.2 | |
| Depreciation and Amortization | | | | | | | | | | |
| Medical | $ | 17.0 | | | | $ | 13.3 | | | $ | 33.3 | | | $ | 15.8 | | | $ | 15.4 | |
| Industrial | 20.1 | | | | 12.4 | | | 49.7 | | | 52.2 | | | 53.7 | |
| Total operating and reportable segments | 37.1 | | | | 25.7 | | | 83.0 | | | 68.0 | | | 69.1 | |
| Corporate and other | 0.2 | | | | 0.2 | | | 0.6 | | | 0.4 | | | 0.4 | |
| Total Depreciation and Amortization | $ | 37.3 | | | | $ | 25.9 | | | $ | 83.6 | | | $ | 68.4 | | | $ | 69.5 | |
The Company’s assets by reportable segment were not included, as this information is not reviewed by, nor otherwise provided to, the chief operating decision maker to make operating decisions or allocate resources.
The following details revenues and property, plant, and equipment, net by geographic region. Revenues generated from external customers are attributed to geographic regions through sales from site locations (in millions).
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Revenues |
| Successor | | | Predecessor |
| From
October 20, 2021
through
December 31, 2021 | | | From July 1, 2021 through
October 19, 2021 | | For Year Ended June 30. 2021 | | For Year Ended June 30. 2020 | | For Year Ended June 30, 2019 |
| North America | | | | | | | | | | |
| Medical | $ | 45.3 | | | | $ | 54.6 | | | $ | 138.6 | | | $ | 57.5 | | | $ | 41.0 | |
| Industrial | 47.6 | | | | 53.4 | | | 199.4 | | | 193.3 | | | 188.3 | |
| Total North America | $ | 92.9 | | | | $ | 108.0 | | | $ | 338.0 | | | $ | 250.8 | | | $ | 229.3 | |
| Europe | | | | | | | | | | |
| Medical | $ | 3.9 | | | | $ | 5.7 | | | $ | 17.1 | | | $ | 5.2 | | | $ | 1.9 | |
| Industrial | 55.3 | | | | 52.6 | | | 241.5 | | | 206.2 | | | 194.9 | |
| Total Europe | $ | 59.2 | | | | $ | 58.3 | | | $ | 258.6 | | | $ | 211.4 | | | $ | 196.8 | |
| Asia Pacific | | | | | | | | | | |
| Medical | $ | — | | | | $ | — | | | $ | — | | | $ | — | | | $ | — | |
| Industrial | 2.0 | | | | 1.7 | | | 15.0 | | | 16.0 | | | 14.0 | |
| Total Asia Pacific | $ | 2.0 | | | | $ | 1.7 | | | $ | 15.0 | | | $ | 16.0 | | | $ | 14.0 | |
| Total revenues | $ | 154.1 | | | | $ | 168.0 | | | $ | 611.6 | | | $ | 478.2 | | | $ | 440.1 | |
Revenues generated in the United States were $86.7 million from the Successor Period October 20, 2021 through December 31, 2021, and were $100.0 million, $306.3 million, $215.5 million, and $198.3 million from the Predecessor Periods from July 1, 2021 through October 19, 2021 and fiscal years ended June 30, 2021, 2020, and 2019, respectively. Revenues in France were $34.4 million from the Successor Period October 20, 2021 through December 31, 2021 and $35.1 million, $158.8 million, $134.5 million, and $128.0 million from the Predecessor Periods from July 1, 2021 through October 19, 2021 and fiscal years ended June 30, 2021, 2020, and 2019, respectively. No other country generated more than 10% of revenue individually.
The following details revenues by timing of recognition (in millions):
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Revenues |
| Successor | | | Predecessor |
| From
October 20, 2021
through
December 31, 2021 | | | From July 1, 2021 through
October 19, 2021 | | For Year Ended June 30. 2021 | | For Year Ended June 30. 2020 | | For Year Ended June 30, 2019 |
| Point in time | $ | 120.1 | | | | $ | 123.6 | | | $ | 456.6 | | | $ | 337.3 | | | $ | 331.1 | |
| Over time | 34.0 | | | | 44.4 | | | 155.0 | | | 140.9 | | | 109.0 | |
| Total revenues | $ | 154.1 | | | | $ | 168.0 | | | $ | 611.6 | | | $ | 478.2 | | | $ | 440.1 | |
The following details revenues by product category (in millions):
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Revenues |
| Successor | | | Predecessor |
| From
October 20, 2021
through
December 31, 2021 | | | From July 1, 2021 through
October 19, 2021 | | For Year Ended June 30. 2021 | | For Year Ended June 30. 2020 | | For Year Ended June 30, 2019 |
| Medical segment: | | | | | | | | | | |
| Medical | $ | 49.2 | | | | $ | 60.3 | | | $ | 155.7 | | | $ | 62.6 | | | $ | 42.9 | |
| Industrial segment: | | | | | | | | | | |
| Reactor Safety and Control Systems | 30.6 | | | | 34.7 | | | 146.8 | | | 135.4 | | | 133.3 | |
| Radiological Search, Measurement, and Analysis Systems | 74.3 | | | | 73.0 | | | 309.1 | | | 280.2 | | | 263.9 | |
| Total revenues | $ | 154.1 | | | | $ | 168.0 | | | $ | 611.6 | | | $ | 478.2 | | | $ | 440.1 | |
The following details property, plant, and equipment, net by geography (in millions):
| | | | | | | | | | | | | | | | | | | | |
| Property, Plant, and Equipment, Net |
| Successor | | | Predecessor |
| As of December 31, 2021 | | | As of June 30, 2021 | | As of June 30, 2020 |
| North America | $ | 77.1 | | | | $ | 47.5 | | | $ | 36.5 | |
| Europe | 46.7 | | | | 41.1 | | | 38.6 | |
| Asia Pacific | 0.2 | | | | 0.2 | | | 0.1 | |
| Total | $ | 124.0 | | | | $ | 88.8 | | | $ | 75.2 | |
17. Fair Value Measurements
The Company applies fair value accounting to all financial assets and liabilities that are recognized or disclosed at fair value in the consolidated financial statements on a recurring basis. The fair value of the Company’s cash and cash equivalents, restricted cash, accounts receivable, and other current assets and liabilities approximates their carrying amounts due to the relatively short maturity of these items. The fair value of third-party notes payable approximates the carrying value because the interest rates are variable and reflect market rates.
Fair Value of Financial Instruments
The Company categorizes assets and liabilities recorded at fair value in the consolidated balance sheets based upon the level of judgment associated with inputs used to measure their fair value. It is not practicable due to cost and effort for the Company to estimate the fair value of notes issued to related parties primarily due to the nature of their terms relative to the entity’s capital structure.
Assets and liabilities carried at fair value are valued and disclosed in one of the following three levels of the valuation hierarchy:
Level 1 – Inputs are unadjusted quoted prices in active markets for identical assets or liabilities.
Level 2 – Inputs are quoted prices in active markets for similar assets or liabilities or inputs that can be corroborated by observable market data for substantially the full term of the assets or liabilities.
Level 3 – Inputs are unobservable and require significant management judgment or estimation.
The following table summarizes the financial assets and liabilities of the Company that are measured at fair value on a recurring basis (in millions):
| | | | | | | | | | | | | | | | | |
| Successor |
| Fair Value Measurements at December 31, 2021 |
| Level 1 | | Level 2 | | Level 3 |
| Assets | | | | | |
| Cash, cash equivalents, and restricted cash (Note 12) | $ | 85.3 | | | $ | — | | | $ | — | |
| Discretionary retirement plan (Note 13) | 3.7 | | | 0.8 | | | — | |
| Liabilities | | | | | |
| Discretionary retirement plan (Note 13) | 3.7 | | | 0.8 | | | — | |
| Public warrants | 46.9 | | | — | | | — | |
| Private placement warrants | — | | | 21.2 | | | — | |
| | | | | | | | | | | | | | | | | |
| Predecessor |
| Fair Value Measurements at June 30, 2021 |
| Level 1 | | Level 2 | | Level 3 |
| Assets | | | | | |
| Cash, cash equivalents, and restricted cash (Note 12) | $ | 102.4 | | | $ | — | | | $ | — | |
| Discretionary retirement plan (Note 13) | 3.4 | | | 0.8 | | | — | |
| Liabilities | | | | | |
| Discretionary retirement plan (Note 13) | 3.4 | | | 0.8 | | | — | |
| Fair Value Measurements at June 30, 2020 |
| Level 1 | | Level 2 | | Level 3 |
| Assets | | | | | |
| Cash, cash equivalents, and restricted cash (Note 12) | $ | 120.0 | | | $ | — | | | $ | — | |
| Discretionary retirement plan (Note 13) | 2.4 | | | 1.1 | | | — | |
| Liabilities | | | | | |
| Discretionary retirement plan (Note 13) | 2.4 | | | 1.1 | | | — | |
As of December 31, 2021, the fair value of Public Warrants issued in connection with GSAH's IPO have been measured based on the listed market price of such Public Warrants, a Level 1 measurement. No Public Warrants or Private Placement Warrants were outstanding as of the June 30, 2021 and June 30, 2020 Predecessor Periods.
As the transfer of Private Placement Warrants to anyone who is not a permitted transferee would result in the Private Placement Warrants having substantially the same terms as the Public Warrants, we determined that the fair value of each Private Placement Warrant is equivalent to that of each Public Warrant. The determination of the fair value of the warrant liability may be subject to change as more current information becomes available and accordingly the actual results could differ significantly. Derivative warrant liabilities are classified as non-current liabilities as their liquidation is not reasonably expected to require the use of current assets or require the creation of current liabilities.
For the period from October 20, 2021 through December 31, 2021, the Company recognized an unrealized gain resulting from an increase in the fair value of the warrant liabilities of $1.2 million, which is presented in the statements of operations as change in fair value of warrant liabilities.
18. Loss Per Share
A reconciliation of the numerator and denominator used in the calculation of basic and diluted loss per common share is as follows (in millions, except per share amounts):
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Successor | | | Predecessor |
| From
October 20, 2021
through
December 31, 2021 | | | From July 1, 2021 through
October 19, 2021 | | Fiscal Year Ended
June 30, 2021 | | Fiscal Year Ended
June 30, 2020 | | Fiscal Year Ended
June 30, 2019 |
| Net loss attributable to Mirion Technologies, Inc. (Successor) / Mirion Technologies (TopCo), Ltd. (Predecessor) shareholders | $ | (22.2) | | | | $ | (105.7) | | | $ | (158.3) | | | $ | (119.1) | | | $ | (122.0) | |
| Weighted average common shares outstanding – basic and diluted | 180.773 | | | | $ | 6.685 | | | $ | 6.549 | | | $ | 6.453 | | | $ | 6.300 | |
| Net loss per common share attributable to Mirion Technologies, Inc. (Successor) / Mirion Technologies (TopCo), Ltd. (Predecessor) — basic and diluted | $ | (0.12) | | | | $ | (15.81) | | | $ | (24.18) | | | $ | (18.45) | | | $ | (19.36) | |
| Anti-dilutive employee share-based awards, excluded | 0.003 | | | 0.200 | | 0.300 | | 0.400 | | 0.500 |
Net loss per share of common stock is computed using the two-class method required for multiple classes of common stock and participating securities based upon their respective rights to receive dividends as if all income for the period has been distributed. Basic loss per share is computed by dividing loss available to common stockholders by the weighted average number of common shares outstanding, adjusted for the outstanding non-vested shares. Diluted loss per share is computed by giving effect to all potentially dilutive securities outstanding for the period using the treasury stock method or the if-converted method based on the nature of such securities. For periods in which the Company reports net losses, diluted net loss per common share attributable to common stockholders is the same as basic net loss per common share attributable to common stockholders, because potentially dilutive common shares are not assumed to have been issued if their effect is anti-dilutive. The Company incurred a net loss for the Successor Period of October 20, 2021 through December 31, 2021 and the Predecessor Periods of July 1, 2021 through October 19, 2021 and the fiscal years ended June 30, 2021, June 30, 2020 and June 30, 2019; therefore, none of the potentially dilutive common shares were included in the diluted share calculations for those periods as they would have been anti-dilutive.
Successor Period
Upon the closing of the Business Combination, the following classes of common stock were considered in the loss per share calculation.
Class A Common Stock
Holders of shares of our Class A common stock are entitled to one vote for each share held of record on all matters on which stockholders are entitled to vote generally, including the election or removal of directors. The holders of our Class A common stock do not have cumulative voting rights in the election of directors. Holders of shares of our Class A common stock are entitled to receive dividends when and if declared by our Board out of funds legally available therefor, subject to any statutory or contractual restrictions on the payment of dividends and to any restrictions on the payment of dividends imposed by the terms of any outstanding preferred stock. Upon our liquidation, dissolution or winding up and after payment in full of all amounts required to be paid to creditors and to the holders of preferred stock having liquidation preferences, if any, the holders of shares of our Class A common stock will be entitled to receive pro rata our remaining assets available for distribution. Class A common stock issued and outstanding is included in the Company’s basic loss per share calculation, with the exception of Founder Shares discussed below.
Class B Common Stock
Holders of shares of our Class B common stock are entitled to one vote for each share held of record on all matters on which stockholders are entitled to vote generally, including the election or removal of directors. If at any time the ratio at
which shares of IntermediateCo Class B common stock are redeemable or exchangeable for shares of our Class A common stock changes from one-for-one as the number of votes to which our Class B common stockholders are entitled will be adjusted accordingly. The holders of our Class B common stock do not have cumulative voting rights in the election of directors. Except for transfers to us pursuant to the IntermediateCo Charter or to certain permitted transferees set forth in our Charter, paired interests may not be sold, transferred or otherwise disposed of.
Holders of shares of our Class B common stock are not entitled to economic interests in us or to receive dividends or to receive a distribution upon our liquidation or winding up. However, if IntermediateCo makes distributions to us other than solely with respect to our Class A common stock, the holders of paired interests will be entitled to receive distributions pro rata in accordance with the percentages of their respective shares of IntermediateCo Class B common stock.
Our Class B common stock has voting rights but no economic interest in the Company and therefore are excluded from the calculation of basic and diluted earnings per share.
Warrants
As described above, the Company has outstanding warrants to purchase up to 27,249,979 shares of Class A common stock. One whole warrant entitles the holder thereof to purchase one share of Mirion Class A common stock at a price of $11.50 per share. The Company’s warrants are not included in the Company’s calculation of basic loss per share and are excluded from the calculation of diluted loss per share because their inclusion would be anti-dilutive.
Founder Shares
Founder shares are subject to certain vesting events and forfeit if a required vesting event does not occur within five years of the closing of the Business Combination. The founder shares are subject to vesting in three equal tranches, based on the volume-weighted average price of our Class A common stock being greater than or equal to $12.00, $14.00 and $16.00 per share for any 20 trading days in any 30 consecutive trading day period. Holders of the founder shares are entitled to vote such founder shares and receive dividends and other distributions with respect to such founder shares prior to vesting, but such dividends and other distributions with respect to unvested founder shares will be set aside by the Company and shall only be paid to the holders of the founder shares upon the vesting of such founder shares.
As the holders of the founder shares are not entitled to participate in earnings unless the vesting conditions are met, the 18,750,000 founders shares have been excluded from the calculation of basic earnings per share. The founders shares are also excluded from the calculation of diluted earnings per share because their inclusion would be anti-dilutive.
Predecessor Period
In the Predecessor Periods presented, the rights, including the liquidation, dividend rights, sharing of losses, and voting rights of Mirion TopCo's A Ordinary Shares B Ordinary Shares were identical. As the rights of both classes of shares were identical, the undistributed earnings are allocated on a proportionate basis and the resulting net loss per share attributed to common stockholders is therefore the same for A Ordinary Shares and B Ordinary Shares on an individual or combined basis.
The Company’s participating securities included the Company’s non-vested A Ordinary Shares, as the holders were entitled to non-forfeitable dividend rights in the event a dividend was paid on ordinary shares. The holders of non-vested A Ordinary Shares did not have a contractual obligation to share in losses.
19. Restructuring
The Company incurs costs associated with restructuring initiatives intended to improve operating performance, profitability, and working capital levels. Actions associated with these initiatives may include improving productivity, workforce reductions, and the consolidation of facilities.
As of December 31, 2021, the Company has identified restructuring actions which will result in additional charges of approximately $1.9 million, primarily in the next 12 months.
The Company’s restructuring expenses are comprised of the following (in millions):
| | | | | | | | | | | | | | | | | |
| Successor |
| From October 20, 2021 through December, 31, 2021 |
| Cost of revenue | | Selling, general
and administrative | | Total |
| Severance and employee costs | $ | 0.1 | | | $ | 0.1 | | | $ | 0.2 | |
Other(1) | — | | | 1.2 | | | 1.2 | |
| Total | $ | 0.1 | | | $ | 1.3 | | | $ | 1.4 | |
| | | | | | | | | | | | | | | | | |
| Predecessor |
| From July 1, 2021 through
October 19, 2021 |
| Cost of revenue | | Selling, general
and administrative | | Total |
| Severance and employee costs | $ | — | | | $ | 1.1 | | | $ | 1.1 | |
Other(1) | 0.1 | | | 0.3 | | | 0.4 | |
| Total | $ | 0.1 | | | $ | 1.4 | | | $ | 1.5 | |
| | | | | |
| For the year ended June 30, 2021 |
| (in millions) | Cost of revenue | | Selling, general
and administrative | | Total |
| Severance and employee costs | $ | 2.4 | | | $ | 1.6 | | | $ | 4.0 | |
Other(1) | 0.7 | | | 0.8 | | | 1.5 | |
| Total | $ | 3.1 | | | $ | 2.4 | | | $ | 5.5 | |
(1) Includes facilities, inventory write-downs, outside services, and IT costs.
The Company does not allocate restructuring charges to segment income; instead, these costs are included in Corporate & other. Restructuring activity and expenses for the fiscal years ended June 30, 2020, and June 30, 2019, were not material.
The following table summarizes the changes in the Company’s accrued restructuring balance, which are included in Accrued expenses and other current liabilities in the accompanying balance sheet (in millions).
| | | | | |
| Predecessor |
| Balance at June 30, 2021 | $ | 3.1 | |
| Restructuring charges | 1.5 | |
| Payments | (2.3) | |
| Adjustments | (0.1) | |
| Balance at October 19, 2021 | $ | 2.2 | |
| |
| Successor |
| Balance at October 20, 2021 | $ | — | |
| Acquisition of Mirion accrued restructuring | 2.2 | |
| Restructuring charges | 1.4 | |
| Payments | (1.8) | |
| Adjustments | (0.4) | |
| Balance at December 31, 2021 | $ | 1.4 | |
20. Noncontrolling Interests
On October 20, 2021, Mirion Technologies, Inc. consummated its previously announced Business Combination pursuant to the Business Combination Agreement.
Before the Closing of the Business Combination, the Sellers had the option to elect to have their equity consideration issued as either shares of Class A common stock or Paired Interests. The Sellers receiving shares of Class B common stock also received one share of IntermediateCo Class B common stock per share of Class B common stock as a Paired Interest. Each of the shares of Class A common stock and each Paired Interest were valued at $10.00 per share for purposes of determining the aggregate number of shares issued to the Sellers. Holders of shares of our Class B common stock are entitled to one vote for each share held of record on all matters on which stockholders are entitled to vote generally, including the election or removal of directors. If at any time the ratio at which shares of IntermediateCo Class B common stock are redeemable or exchangeable for shares of the Company’s our Class A common stock changes from one-for-one, as the number of votes to which our Class B common stockholders are entitled will be adjusted accordingly. The holders of our the Company’s Class B common stock do not have cumulative voting rights in the election of directors. Except for transfers to us pursuant to the IntermediateCo Charter or to certain permitted transferees set forth in our Charter, paired interests may not be sold, transferred or otherwise disposed of.
The holders of IntermediateCo Class B common stock have the right to require IntermediateCo to redeem all or a portion of their IntermediateCo Class B common stock for, at the Company’s election, (1) newly issued shares of the Company’s Class A common stock on a one-for-one basis or (2) a cash payment equal to the product of the number of shares of IntermediateCo Class B common stock subject to redemption and the arithmetic average of the closing stock prices for a share of the Company’s Class A common stock for each of three (3) consecutive full trading days ending on and including the last full trading day immediately prior to the date of redemption (subject to customary adjustments, including for stock splits, stock dividends and reclassifications). This redemption right is available upon the expiration of certain lockup restrictions after April 18, 2022.
At the Closing Date, the Company owned 100% of the voting shares (Class A) of IntermediateCo and approximately 96% of the non-voting Class B shares. The Company recognizes a noncontrolling interest for the 8,560,540 shares, representing approximately 4% of the non-voting Class B shares, of IntermediateCo that are not attributable to the Company.
As of December 31, 2021 noncontrolling interest was $90.8 million reflected in the Consolidated Statements of Stockholders’ Equity (Deficit).
21. Subsequent Events
Subsequent events have been evaluated through February 28, 2022.
ITEM 9. CHANGES IN AND DISAGREEMENTS WITH ACCOUNTANTS ON ACCOUNTING AND FINANCIAL DISCLOSURES
Not applicable.
ITEM 9A. CONTROLS AND PROCEDURES
Evaluation of Disclosure Controls and Procedures
Disclosure controls and procedures are controls and other procedures that are designed to ensure that information required to be disclosed in our reports filed or submitted under the Exchange Act is recorded, processed, summarized and reported within the time periods specified in the SEC’s rules and forms. Disclosure controls and procedures include, without limitation, controls and procedures designed to ensure that information required to be disclosed in our reports filed or submitted under the Exchange Act is accumulated and communicated to our management, including our Chief Executive
Officer and Chief Financial Officer, as appropriate to allow timely decisions regarding required disclosure.
As required by Rules 13a-15 and 15d-15 under the Exchange Act, our Chief Executive Officer and Chief Financial Officer carried out an evaluation of the effectiveness of the design and operation of our disclosure controls and procedures as of December 31, 2021. Based upon their evaluation, our Chief Executive Officer and Chief Financial Officer concluded that, as of December 31, 2021, our disclosure controls and procedures (as defined in Rules 13a- 15(e) and 15d-15(e) under the Exchange Act) are effective.
Management’s Report on Internal Control Over Financial Reporting
Our management is responsible for establishing and maintaining adequate internal control over our financial reporting. As discussed elsewhere in this Form 10-K, we completed the Business Combination on October 20, 2021. Prior to the Business Combination, Mirion Technologies, Inc. was a privately held company and therefore its controls were not required to be designed or maintained in accordance with Exchange Act Rule 13a-15. The design of public company internal controls over financial reporting for the Company following the Business Combination has required and will continue to require significant time and resources from our management and other personnel. Furthermore, GSAH, the legal acquirer in the Business Combination, was a non-operating public shell company prior to the Business Combination, and as such the internal controls of GSAH no longer exist as of the assessment date. As a result, management was unable, without incurring unreasonable effort or expense, to conduct an assessment of our internal control over financial reporting as of December 31, 2021. Therefore, we are excluding management’s report on internal control over financial reporting pursuant to Section 215.02 of the SEC’s Compliance and Disclosure Interpretations. In the future, management’s assessment of our internal control over financial reporting will include an evaluation of such elements as the design and operating effectiveness of key financial reporting controls, process documentation, accounting policies and our overall control environment. In making this assessment, management will use the criteria set forth by the Committee of Sponsoring Organizations of the Treadway Commission (COSO) in Internal Control -- Integrated Framework Scope of the Controls Evaluation (2013 Framework).
Remediation of the Material Weakness in Internal Control Over Financial Reporting
As previously disclosed in GSAH Form 10-K/A filed on May 17, 2021, GSAH identified a material weakness in internal control over financial reporting as of December 31, 2020. A material weakness is a deficiency, or a combination of deficiencies, in internal control over financial reporting, such that there is a reasonable possibility that a material misstatement of the Company’s annual or interim financial statements will not be prevented or detected on a timely basis. Specifically, the Company’s management had concluded that controls around the interpretation and accounting for certain complex features of the Class A common stock and warrants issued by us was not effectively designed or maintained. This material weakness resulted in the restatement of the Company’s financial statements as of and for the year ended December 31, 2020, its balance sheet as of July 2, 2020, and its interim financial statements for the quarter ended September 30, 2020. Additionally, this material weakness could result in a misstatement of the warrant liability, Class A common stock and related accounts and disclosures that would result in a material misstatement of the financial statements that would not be prevented or detected on a timely basis.
Subsequent to the Business Combination on October 20, 2021, and upon filing this Form 10-K for the period ended December 31, 2021, the internal controls over financial reporting of Mirion Technologies, Inc. took the place of the internal controls over financial reporting of GSAH. As a result, the internal control structure of GSAH is no longer in operation. Instead, the relevant internal control structure after completion of the Business Combination is that of Mirion Technologies, Inc. During the Successor Period ended December 31, 2021, we implemented the below changes to our processes to improve our internal control over financial reporting to remediate the control deficiency that gave rise to the material weakness:
•While we have processes to properly identify and evaluate the appropriate accounting technical pronouncements and other literature for all significant or unusual transactions, we have enhanced these processes to ensure that the nuances of such transactions are effectively evaluated in the context of the increasingly complex accounting standards. We require the formalized consideration of obtaining additional technical guidance prior to concluding on all significant or unusual transactions.
•We acquired enhanced access to accounting literature, research materials and documents and increased communication among our personnel and third-party professionals with whom we consult regarding the application of temporary and permanent equity and complex accounting transactions.
After completion of the above changes, our management believes the previously identified material weakness has been remediated.
ITEM 9B. OTHER INFORMATION
Not applicable.
ITEM 9C. DISCLOSURE REGARDING FOREIGN JURISDICTION THAT PREVENT INSPECTIONS
Not applicable.
PART III
ITEM 10. DIRECTORS, EXECUTIVE OFFICERS AND CORPORATE GOVERNANCE
Executive Officers and Directors
Our directors and executive officers and their ages as of the date of this Annual Report are set forth below.
| | | | | | | | |
| Name | Age | Position |
| Thomas D. Logan | 61 | Director, Founder and Chief Executive Officer |
| Brian Schopfer | 37 | Chief Financial Officer |
| Loic Eloy | 45 | President, Group President (Industrial) |
| Lawrence D. Kingsley | 59 | Director and Chairman |
| Jyothsna (Jo) Natauri | 44 | Director |
| Christopher Warren | 46 | Director |
| Steven W. Etzel(1)(2) | 61 | Director |
| Kenneth C. Bockhorst(1)(3) | 49 | Director |
| Robert A. Cascella(2)(3) | 67 | Director |
| John W. Kuo(2)(3) | 58 | Director |
| Jody A. Markopoulos(1)(3) | 50 | Director |
(1) Member of the audit committee.
(2) Member of the compensation committee.
(3) Member of the nominating and corporate governance committee.
Thomas D. Logan currently serves, and has served, as Mirion’s founding Chairman and Chief Executive Officer since 2005, and he has served as a member of Mirion’s board of directors since 2005. Prior to joining Mirion, Mr. Logan served as Chief Executive Officer for Global Dosimetry Solutions, a radiation dosimetry provider, from 2004. Prior to 2004, Mr. Logan served as President of BAF Energy, CFO of E-M Solutions and of BVP, Inc. and prior to that, held various finance leadership positions at Chevron. Mr. Logan has more than 30 years of energy industry experience, as well as extensive experience within the contract manufacturing and consumer products industries. Mr. Logan received a M.B.A. and a B.S. from Cornell University. We believe Mr. Logan’s extensive history with Mirion, as well as his business expertise, qualify him to serve on our Board of Directors.
Brian Schopfer has served as our Chief Financial Officer since 2020. Mr. Schopfer joined Mirion in 2015 and previously served as Mirion’s Executive and Senior Vice President of Business Transformation. In February 2018, Mr. Schopfer left Mirion and joined Omnimax International, a building products company, where he served as Chief Financial Officer of North America until March 2019. Mr. Schopfer rejoined Mirion in March 2019. Prior to joining Mirion, Mr. Schopfer served as Chief Financial Officer for HillPhoenix (part of the Dover Corporation), a commercial refrigeration manufacturer, from 2014 to 2015. Mr. Schopfer also served as the Director of Financial Planning and Analysis for the Dover Corporation, a global manufacturing company, from 2013 to 2014. Mr. Schopfer received a B.S. in Finance and Marketing from the University of Pittsburgh.
Loic Eloy has served as our Industrial Group President since 2022. Mr. Eloy joined Mirion in 2015 and previously served as Vice-President of Mirion’s Detection and Measurement (Health Physics) Division from 2015 to 2019 and President of Mirion’s Radiation Monitoring Systems Division President from 2019 to 2022. Prior to joining Mirion, Mr. Eloy served as Director of Finance and Accounting of Areva from February 2008 to February 2012, and then Commercial Director from February 2012 to January 2015. Prior to 2008, Mr. Eloy held various finance and commercial positions with Siemens. Mr. Eloy received an MBA from the Universidad Panamericana and a Bachelor’s Degree in Finance, Administration, Economics and Marketing from the University of Lyon.
Lawrence D. Kingsley currently serves as the independent Non-Executive Board Chair of IDEXX Laboratories, Inc., a public company, since November 2019 and as an Advisory Director to Berkshire Partners LLC, an investment company, since May 2016. Mr. Kingsley also currently serves as a Director of Polaris Industries Inc., a public company, since January 2016. Prior to joining IDEXX Laboratories, Inc., Mr. Kingsley served as Chairman of Pall Corporation from October 2013 to August 2015 and as President and Chief Executive Officer of Pall Corporation from October 2011 to August 2015 until Danaher Corporation, a public company, acquired Pall Corporation in August 2015. Before his experience at Pall Corporation, Mr. Kingsley served as the Chief Executive Officer and President of IDEX Corporation, a public company specializing in the development, design and manufacture of fluid and metering technologies, health and science technologies and fire, safety and other diversified products, from March 2005 to August 2011, and the Chief Operating Officer of IDEX Corporation from August 2004 to March 2005. Mr. Kingsley previously served as a Director of Pall Corporation from October 2011 to August 2015, Cooper Industries plc (formerly Cooper Industries Ltd.), a public company, from 2007 to 2012 and IDEX Corporation from 2005 to 2011. He was also a director of Rockwell Automation, Inc. from 2013 to 2021. Mr. Kingsley served in various positions of increasing responsibility at Danaher Corporation, including Corporate Vice President and Group Executive from March 2004 to August 2004, President of Industrial Controls Group from April 2002 to July 2004 and President of Motion Group, Special Purpose Systems from January 2001 to March 2002. Mr. Kingsley also previously held management positions of increasing responsibility at Kollmorgen Corporation and Weidmuller Incorporated. Mr. Kingsley received an undergraduate degree in Industrial Engineering and Management from Clarkson University and an M.B.A. from the College of William and Mary. We believe that Mr. Kingsley’s strong executive leadership and operational skills, in-depth knowledge of and experience in strategic planning, corporate development, and operations analysis and experience serving on other public company boards provide him with the qualifications and skills to serve on our Board of Directors.
Jyothsna (Jo) Natauri is a Partner of Goldman Sachs & Co. LLC and has served as the Global Head of Private Healthcare Investing within Goldman Sachs Asset Management since May 2018. Prior to assuming her current role, Ms. Natauri was an investment banker with Goldman Sachs for 12 years, where she led coverage of large cap companies in healthcare and other industries. She was named managing director in 2008 and partner in 2012. Ms. Natauri has served as a director on the board of Flywire Corporation since November 2020, and also serves on the boards of MyEyeDr, Sita Foundation and Safe Horizon. She previously served on the board of Avantor from November 2018 to May 2021. Ms. Natauri received a B.A. from the University of Virginia in Economics and Biology. We believe that Ms. Natauri’s experience of over 20 years in covering companies and executing transactions provides her with the qualifications and skills to serve on our Board of Directors.
Christopher Warren currently serves, and has served, as a partner at Charterhouse Capital Partners LLP since he joined in 2013. Prior to joining Charterhouse, Mr. Warren served as a partner at ECI Partners, a private equity group, from 2003 to 2013. He also served as Associate at BC Partners, an international investment firm, and as Consultant at COBA, a UK-based strategy consulting firm. Mr. Warren received a Master of Arts in Philosophy, Politics and Economics from Oxford University and an MBA from INSEAD. We believe that Mr. Warren’s extensive business experience provides him with the qualifications and skills to serve on our Board of Directors.
Steven W. Etzel has served as Senior Vice President and Chief Financial Officer of Rockwell Automation, Inc., a company focused on industrial automation and information, from November 2020 to February 2021, and subsequently as Senior Vice President, Finance of Rockwell until his retirement in April 2021. Mr. Etzel joined Rockwell in 1989 and served in various positions, including Vice President and Treasurer from 2007 to 2020 and Vice President, Finance from October 2020 to November 2020. Mr. Etzel received his Bachelor of Science degree in Business Administration from Clarion University of Pennsylvania. We believe Mr. Etzel’s extensive financial and management experience, including financial reporting, internal controls, investor relations, financial planning and analysis, capital markets financing transactions, mergers and acquisitions and risk management provides him with the qualifications and skills to serve on our Board of Directors.
Kenneth C. Bockhorst currently serves as the Chairman, President and Chief Executive Officer of Badger Meter, Inc., a global provider of industry leading smart water solutions that optimize operations and enhance sustainability across a wide range of customer applications. Mr. Bockhorst joined Badger Meter in October 2017 as Chief Operating Officer, was promoted to President in April 2018, Chief Executive Officer in 2019 and Chairman of the Board in 2020. Prior to Badger Meter, he served six years at Actuant Corporation, a diversified industrial company (now named Enerpac Tool Group), most recently as Executive Vice President of the Energy segment. Prior to Actuant, he held product management and operational leadership roles at IDEX and Eaton. Mr. Bockhorst received an M.B.A. from the University of Wisconsin - Madison and a B.A. from Marian University in Operations Management, Marketing and Human Resources. We believe Mr. Bockhorst's extensive operational experience with diversified industrial companies provides him with the qualifications and skills to serve on our Board of Directors.
Robert A. Cascella effective as of December 31, 2021 retired from the position of Strategic Business Development Leader for Royal Philips, a public Dutch healthcare company and has held this position since May 2020. From April 2015 to April
2020, he served as Executive Vice President and Chief Business Leader of Philips’ Diagnosis and Treatment and Precision Diagnosis businesses. He also served on Philip’s Executive Committee from January 2016 to April 2021. Prior to Philips, Mr. Cascella served at Hologic, Inc., a public medical device and diagnostics company, from February 2003 to December 2013 as its president and later CEO. He has also held senior leadership positions at CFG Capital, NeoVision Corporation and Fischer Imaging Corporation. Mr. Cascella has served as the chair of the board of Neuronetics, Inc. since April 2021, on the board of Metabolon, Inc. since September 2020 and on the board of Celestica Inc. since April 2019, where he has also served as chair of the compensation committee since July 2021. He previously served on the board of Tegra Medical and acted as chair of the boards of Dysis Medical and Miranda Medical. Mr. Cascella received a B.A. in accounting from Fairfield University. We believe Mr. Cascella's extensive medical device and healthcare business experience, as well as his experience serving on other public company boards, provide him with the qualifications and skills to serve on our Board of Directors.
John W. Kuo is the Chief Legal Officer of Visby Medical, a privately-held molecular diagnostic company, and has held such position since September 2021. Previously, he was the Executive Vice President, General Counsel, Chief Compliance Officer and Corporate Secretary of Charles River Laboratories, a NYSE-listed, Fortune 1000 global contract drug research and development company, from May 2020 to September 2020. Before that, Mr. Kuo was the Senior Vice President, General Counsel and Corporate Secretary of Varian Medical Systems, a NYSE-listed, Fortune 1000 global cancer therapy/radiation therapy company, from July 2005 to May 2020. He also previously served in senior expatriate roles in Europe and Asia in the energy and high technology industries, respectively. Mr. Kuo received his J.D. from the University of California, Berkeley School of Law and his B.A. in Biology & Society from Cornell University. We believe that Mr. Kuo’s over 15 years of experience as an executive in Fortune 1000 life sciences companies, his familiarity with the radiation therapy industry, his global perspective and international market expansion experience, his deep understanding of regulated industries, his management experience in scaling global functions and his expertise in legal and corporate governance matters provide him with the qualifications and skills to serve on our Board of Directors.
Jody A. Markopoulos is currently consulting. Previously, Ms. Markopoulos served as the Chief Operating Officer of Eos Energy Enterprises, Inc., a producer of low-cost battery storage solutions for the electric utility industry from March 2021 to November 2021. Previously, she spent 26 years in multiple operating leadership roles at General Electric and Baker Hughes. She served as the Chief Supply Officer at Baker Hughes, a GE company responsible for supply chain operations, from 2017 to 2018, and then as Chief Transition Officer from 2018 to 2020 responsible for executing the orderly transition from GE. At General Electric, she served as Chief Operations Officer at GE Oil & Gas from 2015 to 2017, President and CEO of GE Intelligent Platforms from 2011 to 2014 and as Vice President of Sourcing at GE Energy from 2005 to 2011. Ms. Markopoulos received a B.S. in Interdisciplinary Engineering and Management from Clarkson University. We believe Ms. Markopoulos’s experience as an operating executive qualifies her to serve as a director on our Board of Directors.
Family Relationships
There are no family relationships among any of the individuals who serve as directors or executive officers of Mirion.
Board of Directors
Our business and affairs are organized under the direction of the Board of Directors. Lawrence D. Kingsley serves as Chairman of the Board of Directors. The primary responsibilities of the Board of Directors is to provide oversight, strategic guidance, counseling and direction to management. The Board of Directors meets on a regular basis and additionally, as required.
In addition, we are a party to a director nomination agreement with certain entities affiliated with Charterhouse and a director nomination agreement with the Sponsor that provide Charterhouse with the right to nominate one director to our Board and the Sponsor to nominate two directors to our Board, subject to certain fallaway provisions. See “Certain Relationships and Related Transactions, and Director Independence—Director Nomination Agreements” for more information.
Each of our current directors will continue to serve until the election and qualification of his or her successor, or his or her earlier death, resignation or removal.
Role of Board in Risk Oversight
Our Board has extensive involvement in the oversight of risk management related to us and our business and accomplishes this oversight through the regular reporting to the Board by the audit committee. The audit committee represents the Board by periodically reviewing our accounting, reporting and financial practices, including the integrity of our financial statements, the monitoring of administrative and financial controls and our compliance with legal and regulatory requirements. Through its regular meetings with management, including the finance, legal, internal audit and information
technology functions, the audit committee reviews and discusses all significant areas of our business and summarizes for our Board all areas of risk and the appropriate mitigating factors. In addition, our Board receives periodic detailed operating performance reviews from management.
Board Committees
Our Board has an audit committee, a compensation committee and a nominating and corporate governance committee, each of which will has the composition and responsibilities described below. Members serve on these committees until their resignation or until otherwise determined by the Board.
Audit Committee
Our Board’s audit committee consists of Steven W. Etzel, Kenneth C. Bockhorst and Jody A. Markopoulos, with Steven W. Etzel serving as the chair of the committee. Our Board has determined that Steven W. Etzel, Kenneth C. Bockhorst and Jody A. Markopoulos are “independent” as defined under applicable NYSE listing standards, including the standards specific to members of an audit committee, and Rule 10A-3 of the Exchange Act, and are financially literate.
Our Board has also determined that Steven W. Etzel qualifies as an audit committee financial expert within the meaning of SEC regulations and meets the financial sophistication requirements of the NYSE listing rules. In making this determination, our Board considered Steven W. Etzel’s formal education and previous and current experience in financial and accounting roles. Our independent registered public accounting firm and management periodically will meet privately with the audit committee.
The audit committee is responsible for, among other things:
•appointing, compensating, retaining, evaluating, terminating and overseeing our independent registered public accounting firm;
•discussing with our independent registered public accounting firm their independence;
•reviewing with our independent registered public accounting firm the scope and results of their audit;
•approving all audit and permissible non-audit services to be performed by our independent registered public accounting firm;
•overseeing the financial reporting process and discussing with management and our independent registered public accounting firm the interim and annual financial statements that we file with the SEC;
•reviewing our policies on risk assessment and risk management;
•reviewing related party transactions;
•designing and implementing the internal audit function;
•overseeing our financial and accounting controls and compliance with legal and regulatory requirements; and
•establishing procedures for the confidential anonymous submission of concerns regarding questionable accounting, internal controls or auditing matters.
Compensation Committee
Our Board’s compensation committee consists of Robert A. Cascella, Steven W. Etzel and John W. Kuo, with Robert A. Cascella serving as the chair of the committee. Robert A. Cascella, Steven W. Etzel and John W. Kuo are non-employee directors, as defined in Rule 16b-3 promulgated under the Exchange Act. Our Board has determined that Robert A. Cascella, Steven W. Etzel and John W. Kuo are “independent” as defined under applicable NYSE listing standards, including the standards specific to members of a compensation committee.
The compensation committee is responsible for, among other things:
•determining, or recommending to our Board for determination, the compensation of our executive officers, including the chief executive officer;
•administering our equity compensation plans;
•overseeing our overall compensation policies and practices, compensation plans, and benefits programs; and
•appointing and overseeing any compensation consultants.
We believe that the composition and functioning of the compensation committee meets the requirements for independence under applicable NYSE listing standards.
Nominating and Corporate Governance Committee
Our Board’s nominating and corporate governance committee consists of John W. Kuo, Kenneth C. Bockhorst, Robert A. Cascella and Jody A. Markopoulos, with John W. Kuo serving as the chair of the committee. Our Board has determined that each of these individuals is “independent” as defined under applicable SEC rules and NYSE listing standards.
The nominating and corporate governance committee is responsible for, among other things:
•evaluating and making recommendations regarding the composition, organization and governance of our Board and its committees;
•reviewing and making recommendations with regard to our corporate governance guidelines and compliance with laws and regulations; and
•overseeing an evaluation of our Board and its committees.
We believe that the composition and functioning of the nominating and corporate governance committee meets the requirements for independence under current NYSE listing standards.
The audit, compensation, and nominating and corporate governance committees each operate under a written charter that satisfies the applicable rules and regulations of NYSE and the SEC.
We have posted the charters of our Board’s audit, compensation and nominating and corporate governance committees, and we intend to post any amendments thereto that may be adopted from time to time, on our website. Information on or that can be accessed through our website is not part of this Annual Report. Our Board may from time to time establish other committees.
Code of Ethics and Business Conduct
We have adopted a Code of Ethics and Business Conduct that applies to all of our employees, officers, and directors, including our Chief Executive Officer, Chief Financial Officer, and other executive and senior financial officers. The full text of our Code of Ethics and Business Conduct is available on the investor relations page on our website, ir.mirion.com. Information on, or that can be accessed through, our website is not part of this Annual Report.
Governance Documents
We believe that good corporate governance is important to ensure that Mirion is managed for the long-term benefit of our stockholders. Our Nominating and Governance Committee will periodically review and reassess our Corporate Governance Guidelines and overall governance structure. Complete copies of our current Board committee charters and our Corporate Governance Guidelines and our Code of Business Conduct and Ethics are available on our investor relations website, ir.mirion.com. Information on, or that can be accessed through, our website is not part of this Annual Report.
Compensation Committee Interlocks and Insider Participation
None of our executive officers currently serves, or has served during the last year, as a member of the board of directors or compensation committee of any entity that has one or more executive officers serving as a member of our Board.
ITEM 11. EXECUTIVE COMPENSATION
Overview
This Compensation Discussion and Analysis describes our executive compensation program in fiscal 2021 for our named executive officers (“NEOs”), including philosophy, process, objectives and the elements of the program and the material factors considered in making compensation decisions. Our NEOs for the period from July 1, 2020 through December 31, 2021 are listed in the table below.1
| | | | | |
| Name | Position |
| Thomas Logan | Chief Executive Officer |
1 We only had three (3) executive officers during the period from July 1, 2020 through December 31, 2021, accordingly, we only have disclosure with respect to (3) Named Executive Officers.
| | | | | |
| Brian Schopfer | Chief Financial Officer |
| Michael Freed (1) | Chief Operating Officer |
(1) Mr. Freed, who has served as the Chief Operating Officer of the Company, will depart the Company on February 28, 2022 as a result of his position being eliminated.
Business Highlights
Successful Business Combination
On June 17, 2021, GSAH announced a signed merger agreement that resulted in the combination of GSAH and Mirion Technologies, Inc. The Business Combination closed on October 20, 2021 and Mirion began trading thereafter as a public operating company on the NYSE. The consideration paid to Mirion sellers in connection with the Business Combination was approximately $1.3 billion in cash, 30,401,902 million newly issued shares of Class A common stock and 8,560,540 million newly issued shares of the Class B common stock. At the same time as of the Business Combination, GSAH closed on a $900.0 million investment in which investors paid a $10 per share price for our Class A common stock (the "Pipe Investment Price").
New Credit Agreement
On October 20, 2021, the Company entered into the 2021 Credit Agreement that provides for an $830.0 million senior secured first lien term loan facility and a $90.0 million senior secured revolving facility and extinguished the 2019 Credit Facility.
CIRS Acquisition
On December 1, 2021, the Company purchased 100% of the issued and outstanding shares of CIRS for an aggregate of $54 million, net of cash acquired of $1.0 million. CIRS is a leading provider of medical imaging and radiation therapy phantoms serving the medical industry located in the United States. The acquisition is included in our Medical segment and will advance the Company’s strategy to further expand into the medical treatment markets globally.
Other Acquisitions
Mirion completed three acquisitions of U.S.-based providers of dosimetry services which we believe will increase the US footprint of Mirion's industry-leading dosimetry product offering. On December 1, 2021 we acquired Safeline Monitors Systems LLC, for $1.5 million plus contingent consideration of $0.5 million. On November 1, 2021, we acquired CHP Dosimetry, for $2.5 million. On September 1, 2021 we acquired Dosimetry Badge for $1.8 million plus contingent consideration of $0.8 million.
Financial Highlights
•Our revenues were $154.1 million for the Successor Period and $168.0 million for the Predecessor Stub Period, of which 31.9% and 35.9% was generated in the Medical segment for the Successor and Predecessor Stub Periods, respectively, and 68.1% and 64.1% was generated in the Industrial segment for the Successor and Predecessor Stub Periods, respectively
•Backlog (representing committed but undelivered contracts and purchase orders, including funded and unfunded government contracts) was $747.5 million and $715.8 million as of December 31, 2021, and June 30, 2021, respectively
•GAAP net loss for the successor period of October 20, 2021 to December 31, 2021 was $23.0 million and for the predecessor period of July 1, 2021 to October 19, 2021 was $105.7 million
•Adjusted EBITDA for the Successor Period of October 20, 2021 to December 31, 2021 was $44.5 million and for the Predecessor Period of October 1, 2021 to October 19, 2021 was $31.2 million
•The company initiated calendar year 2022 guidance of 5% to 7% organic adjusted revenue growth and adjusted EBITDA of $175 million to $185 million.
Compensation Philosophy and Objectives
Through our Compensation Committee we have adopted a Total Rewards Philosophy designed to guide the development of a total compensation package that attracts, motivates and retains high quality executives needed to fulfill our mission. Our goal is to support business priorities deemed essential by our Board of Directors, as well as satisfy the accepted governance standards of impartial external stakeholders. We have designed compensation packages that (i) are competitive with market practice, (ii) reward both organizational and individual performance and (iii) closely align the interests of our NEOs with those of our stockholders by providing a significant portion of our NEOs’ compensation in equity. We use this philosophy as the foundation for evaluating and implementing our executive compensation program, with a heavy emphasis on pay that is variable or at risk depending directly on performance against strategic corporate metrics, in other words, paying for performance.
Our executive compensation program framework since the Business Combination includes a mix of three key compensation elements—(i) base salary, (ii) short-term cash incentive awards and (iii) long-term equity incentive awards. In determining the amount of each compensation element awarded to our NEOs, our Compensation Committee looks at each NEO’s overall compensation package, as well as the amount of each compensation element for the NEO relative to both internal and external pay to determine whether such amounts and the overall mix of elements for the NEO’s role further the principles and objectives of our executive compensation program.
The Compensation Committee will annually review and analyze market trends and adjust the design and operation of our executive compensation program from time to time as it deems necessary and appropriate. In structuring and adjusting the executive compensation program, the Compensation Committee places no formal weighting on any one factor. As we continue to mature as a public company, the Compensation Committee will continue to review and evaluate our executive compensation program to ensure it aligns with our compensation philosophy and objectives.
Executive Compensation Best Practices
In executing our compensation program and determining executive compensation, we are guided by the following corporate governance best practices designed to protect the interests of our stockholders. As we transition from a new public company to a more mature public company, we will continue to evaluate our compensation program relative to our third-party developed peers group.
| | | | | |
| What We Do | What We Don’t Do |
☒ Pay-for-Performance Philosophy. We align pay and performance by awarding a substantial portion of the compensation paid to our executives in the form of variable, “at-risk” performance-based compensation linked to achievement of rigorous performance goals. ☒ Balanced Short-Term and Long-Term Compensation. We grant compensation that discourages short-term risk taking at the expense of long-term results ☒ Maintain an Independent Compensation Committee and Independent Compensation Committee Advisor. Our Compensation Committee is comprised solely of independent directors and engages its own independent consultant. ☒ Share Ownership Guidelines. All NEOs are subject to significant share ownership guidelines. Pursuant to our Share Ownership Guidelines, our CEO is required to hold 5x base salary, our CFO is required to hold 3x base salary and the other members of our executive leadership team are required to hold 1x base salary. ☒ Clawback. The Board will require reimbursement to the Company of any performance-based award in the event of certain accounting restatements due to the material noncompliance of the Company with any financial reporting requirement under the securities laws. Our clawback policy is described in more detail under “Other Compensation Governance Practices – Clawback Policy” below. | ☒ No Excise Tax “Gross-Ups”. We do not provide any “gross-ups” for excise taxes that our employees might owe as a result of the application of Sections 280G or 4999 of the IR ☒ No “Single-Trigger” Change in Control Arrangements. We do not provide for “single-trigger” acceleration of compensation or benefits solely upon a change in control ☒ No Excessive Perks. We generally do not provide any excessive perquisites to our NEOs ☒ Do Not Permit Hedging or Pledging. We prohibit directors and employees, including our NEOs, from hedging and pledging our securities |
Executive Compensation Process
Role of the Compensation Committee and Management
With respect to the portion of fiscal 2021 that preceded the closing of the Business Combination, our executive compensation program was administered by the Remuneration Committee of the Board of Directors of Mirion Technologies (Topco), Ltd. (the "Remuneration Committee"), which was controlled by our former controlling shareholder, (Charterhouse Capital Partners), with executive compensation reviewed annually and other compensation matters reviewed on an ad hoc basis.
Since the closing of the Business Combination, our executive compensation program has been administered by our Compensation Committee, which is comprised entirely of independent directors. Our Compensation Committee is responsible for establishing, implementing, monitoring and evaluating our executive compensation program. The Compensation Committee reviews and approves the compensation of our NEOs, other than the compensation of our CEO, for which the Compensation Committee makes recommendations to our Board. The Compensation Committee’s responsibilities and authority are described fully in the Compensation Committee’s charter, which is available on our website at www.mirion.com (under the tab “Investors” and under the subtab “Governance —Governance Documents”).
The Compensation Committee also consults with members of our management team, including our CEO, and our chairperson when making compensation decisions. While the Compensation Committee considers our CEO’s recommendations, the Compensation Committee ultimately uses its own business judgment and experience in approving, or making recommendations to the Board where applicable, regarding individual compensation elements and the amount of each element for our NEOs. Our CEO recuses himself from all determinations regarding his own compensation.
The compensation of our NEOs will be reviewed at least annually by our Compensation Committee and will be informed by the recommendations of our CEO (other than with respect to his own compensation) and our compensation consultant. Our Compensation Committee will then evaluate and determine any recommended compensation adjustments or awards to our NEOs or make recommendations to the Board for final determination.
Role of Compensation Consultant
Following the closing of the Business Combination, the Compensation Committee engaged Willis Towers Watson to be its compensation consultant and assist on matters relating to our executive compensation program pursuant to its authority
under the Compensation Committee charter. A representative of Willis Towers Watson attended meetings of the Compensation Committee as requested. Willis Towers Watson reports directly to the Compensation Committee.
The Compensation Committee has evaluated Willis Towers Watson’s independence by considering the requirements mandated by NYSE listing standards and SEC rules and has determined that no conflict of interest exists. The Compensation Committee is directly responsible for the appointment, compensation and oversight of the work of any such advisor and has sole authority to approve all such advisors’ fees and other retention terms. Willis Towers Watson has not provided any other services to us and has received no compensation other than with respect to the services described above.
Peer Group
The Compensation Committee, with the guidance of Willis Towers Watson, developed and approved the following compensation peer group in December 2021. The establishment of this peer group is to provide a comparative basis for the compensation of our executive officers to their peer companies in order to set appropriate overall compensation framework.
Peer Group Companies
| | | | | | | | |
| Allied Motion Technologies Inc. | Bruker Corporation | Proto Labs, Inc. |
| Array Technologies, Inc. | Coherent, Inc. | Raven Industries, Inc. |
| Babcock & Wilcox Enterprises, Inc. | Graco, Inc. | Repligen Corporation |
| Badger Meter, Inc. | MSA Safety Incorporated | Sotera Health Company |
| Bio-Techne Corporation | Nordson Corporation | Vicor Corporation |
Our peer group is designed to reflect both business competitors and competitors for talent. Peers consist of two industries, Industrial and Health Care, at approximately a two-thirds, one-third mix, respectively, commensurate with our revenues from each industry. Peers reflect similarly sized organizations based on trailing twelve month revenues of between one-third to three times our trailing twelve month revenues at the time of the analysis.
The Compensation Committee references the market data of the peer group as a guide when making decisions. Market data is one element that the Compensation Committee uses to make pay decisions. Multiple factors are considered in determining total compensation opportunity, including our compensation philosophy, the executive’s role and responsibility, the executive’s past performance, internal equity, and expected contributions and experience in the role.
The Compensation Committee will review our compensation peer group at least annually and make adjustments to its composition as necessary or appropriate, taking into account changes in both our business and the businesses of the companies in the compensation peer group.
Analysis of Fiscal 2021 Compensation
Compensation Elements
The 2021 executive compensation program consisted of the following elements: base salary, annual incentive compensation and long-term equity incentive compensation in the form of restricted stock units (“RSUs”), performance stock units (“PSUs”) and, for Mr. Logan and Mr. Schopfer, Profits Interests. Each element, which is further discussed below, is intended to reward and motivate executives in different ways consistent with our overall compensation philosophy. Each of the above-described compensation elements for our NEOs for fiscal 2021 is discussed in detail below, including a description of the particular element and how it fits into our overall executive compensation philosophy and objectives.
Base Salary
The NEOs receive a base salary to compensate them for services rendered and reward them for their performance. The base salary payable to each NEO is intended to provide a fixed component of compensation reflecting the executive’s skill set, experience, role, and responsibilities. We believe that a competitive base salary is a necessary element of our executive compensation program and is critical in attracting and retaining executive talent, including our NEOs. Base salaries for our NEOs are also intended to be competitive with those received by other individuals in similar positions at the companies with which we compete for talent, as well as equitable internally across our executive team.
Each NEO’s initial base salary was provided in his employment agreement and was reviewed and, if appropriate, adjusted on an annual basis. In December 2021, the Compensation Committee reviewed the base salaries of our NEOs, taking into consideration a competitive market analysis performed by Willis Towers Watson. Consistent with our intended approach to provide compensation competitive with peer group companies and in recognition of their performance, the Compensation Committee approved an increase in the annual base salaries for Messrs. Logan and Schopfer effective as of December 27, 2021. The base salaries for our NEOs both before and after the increase are set forth in the table below. The actual base salaries paid to each NEO for fiscal year 2021 are set forth above in the 2021 Summary Compensation Table in the column entitled “Salary.”
Fiscal 2021 Base Salaries
| | | | | | | | | | | |
| Name | Base Salary Prior to December 27, 2021
($) | Base Salary Effective as of December 27, 2021
($) | Change (%) |
| Mr. Logan | 660,000 | 700,000 | 6.1 |
| Mr. Schopfer | 396,000 | 450,000 | 13.6 |
| Mr. Freed | 412,000 | 412,000 | - |
Going forward, the Compensation Committee will review the base salaries of our NEOs annually and make adjustments to base salaries as it determines to be necessary or appropriate. To the extent base salaries are adjusted, the amount of any such adjustment would reflect a review of competitive market data, consideration of relative levels of pay internally, individual performance of the NEO, and any other circumstances that the Compensation Committee determines are relevant. Due to their salary increases in December 2021, none of the NEOs is eligible for a salary increase during the first quarter of 2022.
Short-Term Incentive Compensation
Fiscal 2021 (July 1, 2020 through June 30, 2021) Short-Term Incentive Program
Prior to the October 20, 2021 Business Combination, we maintained a cash-based annual incentive program for executives, including the NEOs, in which such executives were eligible to receive cash bonuses based on achievement of specified performance goals. Such awards were designed to incentivize the NEOs with a variable level of compensation based on performance measures established by the Remuneration Committee (and, for executives other than Mr. Logan, by Mr. Logan) that were tied to predefined business and personal goals and objectives.
In fiscal year 2021, Messrs. Logan, Schopfer and Freed were eligible to earn annual cash bonuses targeted at 80%, 50% and 50%, respectively, of their base salaries (the "FY'21 Incentive Plan"). Each NEO was eligible to earn his bonus based on the attainment of business unit and personal goals and objectives, set and approved by the Remuneration Committee and Mr. Logan prior to the Business Combination (Mr. Logan’s target bonus was set and approved by the Remuneration Committee alone). Bonus amounts under the FY’21 Incentive Plan bonus pool were determined based on achievement of
Adjusted EBITDA goals for each participant’s business unit(s) with an additional modifier based on our Free Cash Flow
(determined on a consolidated basis), that may increase or decrease the size of a participant’s award. After calculating
awards based on these objective financial metrics, 35% of the award was subject to additional specified personal business
objectives determined by Mr. Logan (for NEOs other than Mr. Logan) in consultation with the Remuneration Committee,
and for Mr. Logan it was determined by the Remuneration Committee alone.
In September 2021, the Remuneration Committee (which was controlled by our former controlling shareholder, Charterhouse) considered the performance goals set forth above and the overall performance of each of the NEOs during fiscal year 2021 and determined that the total amount of the cash bonuses to be paid to the NEOs with respect to fiscal year 2021 equaled the target amount of their respective FY’21 Incentive Plan opportunity. Accordingly, the Remuneration Committee determined that the actual payments for the NEOs pursuant to the FY’21 Incentive Plan for Messrs. Logan, Schopfer and Freed were $511,410, $177,613 and $199,425, respectively.
Stub 2021 Short-Term Incentive Program
In anticipation of transitioning to a calendar year reporting company beginning in 2022, we adopted a six-month short-term incentive program effective from July 1, 2021 through December 31, 2021 (the “Stub 2021 Incentive Plan”),
intended to provide cash incentive compensation opportunities to our officers (including the NEOs) and employees in the
interim period beginning after the end of the FY’21 Incentive Plan performance period and before the 2022 STIP (as
discussed further below) went into effect. Under the Stub 2021 Incentive Plan, Messrs. Logan, Schopfer and Freed were
eligible to earn annual cash bonuses targeted at 80%, 50% and 50%, respectively, of their base salaries. Bonus amounts
under the Stub 2021 Incentive Plan were determined based on achievement of Adjusted EBITDA goals on an enterprise
wide basis, with a target Adjusted EBITDA of $81,305,000.
The Compensation Committee reviewed the performance with respect to the performance goals set forth above and determined that the Adjusted EBITDA, on a consolidated basis, was achieved at $76,612,000 or 94.2% of target. Accordingly, the Compensation Committee determined that the actual payments for the NEOs pursuant to the Stub
2021 Incentive Plan for Messrs. Logan, Schopfer and Freed were $174,736, $71,038 and $72,300, respectively.
Executive STIP for 2022
On December 27, 2021, the Compensation Committee adopted and approved the terms of the Company’s executive short-term incentive compensation program (the “STIP”) for 2022, which will govern the terms of annual cash incentive awards granted to eligible executives of the Company (including each of the Company’s current NEOs) going forward. Payments under the STIP are based on the achievement of specified performance goals, including 50% on Adjusted EBITDA, 30% on Organic Revenue Growth and 20% on Free Cash Flow. The specific targets relating to the performance goals will be set in connection with the establishment of the Company’s budget for the 2022 calendar year. In connection with its
review and approval of the STIP, our Compensation Committee reviewed the target incentive opportunities of our NEOs,
taking into consideration a competitive market analysis performed by Willis Towers Watson. Consistent with our intended
approach to provide compensation competitive with peer group companies and in recognition of their performance, the
Compensation Committee approved the following target incentive opportunities (expressed as a percentage of base salary)
for our NEOs for fiscal year 2022: Mr. Logan – 100%; Mr. Schopfer – 65%; and Mr. Freed – 50%.
Michael Freed Short-Term Incentive Bonus
On December 27, 2021, the Compensation Committee approved a special short-term incentive bonus to Mr. Freed with a target value of $150,000, to be paid in two installments subject to (i) the achievement of Adjusted EBITDA results based on the Company's budgeted performance as measured on June 30, 2022 and December 31, 2022, and (ii) his continued employment through each of the applicable payment dates. This special short-term incentive is in addition to Mr. Freed's annual Executive STIP opportunity. Because Mr. Freed departed Mirion on February 28, 2022, no portion of this special short-term incentive bonus will be paid.
Long-Term Equity Incentive Compensation
We believe that providing long-term incentive compensation in the form of equity awards is a critical element of our executive compensation program as it reinforces our pay-for-performance culture and aligns employees’ interests and contributions with the long-term interests of the Company’s stockholders. In addition, our Compensation Committee and Board believe that offering meaningful equity ownership in the Company is helpful in retaining our NEOs and other key employees.
We adopted and obtained stockholder approval for the 2021 Omnibus Incentive Plan (the “Incentive Plan”) in connection with the Business Combination and, in December 2021, we granted certain of our executive officers, including Mr. Logan and Mr. Schopfer, restricted stock units (“RSUs”) and performance stock units (“PSUs”) pursuant to the Incentive Plan.
The table below sets forth the RSUs and PSUs granted to Messrs. Logan and Schopfer during 2021. These initial grants
(the “Bridge RSUs” and “Bridge PSUs” and, collectively, the “Bridge Grants”) under the Incentive Plan were intended, as
a retention device, to bridge the gap from the time the Incentive Plan was adopted in December 2021 until such time that
the Company is in a position to make its first in-cycle annual grants under the Incentive Plan in 2022. In addition to
assisting retention, the Bridge Grants were intended to reward the extraordinary efforts of certain officers and employees,
including Messrs. Logan and Schopfer, in connection with executing the Business Combination.
Equity Awards Granted in Fiscal 2021
| | | | | | | | |
| NEO | RSUs Granted
(#) | PSUs Granted (Target)
(#) |
| Mr. Logan | 381,679 | 95,419 |
| Mr. Schopfer | 76,355 | 19,083 |
The RSUs will vest in four equal annual installments beginning on the first anniversary of the grant date, and the PSUs will vest following the end of a three-year performance period subject to the achievement of specified performance goals in respect of relative total stockholder return (“Relative TSR”) and organic revenue growth as described in more detail below.
PSUs
The PSUs provide the right to receive shares of our common stock at a future date, assuming performance against pre-determined metrics are achieved, specifically Relative TSR and organic revenue growth which are equally weighted 50% each for both Mr. Logan and Mr. Schopfer. The number of PSUs that may be achieved are capped at 100% of target with the value ultimately received based on our performance against these metrics, which are key measures of our long-term performance, as well as the growth of the price of our share of common stock over time. Subject to continued employment through the end of the performance period and achievement of minimum performance criteria, the PSUs will be eligible to vest in 2025 following the date that the Compensation Committee certifies the Company’s achievement of the performance goals (as described below) following December 31, 2024, the final day of the performance period.
Total Shareholder Return Metrics
Relative TSR comprises 50% of the total PSU award granted to both Mr. Logan and Mr. Schopfer. The Relative TSR performance period is three years, from January 1, 2022 through December 31, 2024, and is measured as compared to the Russell 2000 Industrials peers. The Russell 2000 Industrial index was chosen because the Company is transitioning the focus of its business from the industrial market to the healthcare market. In future years, the Compensation Committee will reevaluate the index and associated composition. The following targets were set with respect to the Relative TSR metric:
| | | | | | | | | | | | | | | | | | | | |
| | Percentile | | Payout |
| Below Threshold | | <30% | | 0% |
| Threshold | | 30% | | 25% |
| Target | | 55% | | 50% |
| Maximum | | ≥80% | | 100% |
The payout in respect of these performance shares will be made in shares of our common stock and/or cash in an amount determined based on the TSR of our common stock, assuming reinvestment of all dividends, compared to the performance of companies in the Russell 2000 Index for the period from January 1, 2022 to December 31, 2024, if the individual continues as an employee until the third anniversary of the grant date (subject to provisions relating to the grantee’s death, disability or retirement or a change of control of the Company). We use the 20-trading day average trading price of our common stock ending December 31 to determine the starting price and the final TSR. The potential value of a payout will fluctuate with the market value of our common stock. The percentage of the Relative TSR PSUs that vest will be interpolated, on a mathematical straight-line basis. In no event will participants be eligible to receive more than 100% of the Relative TSR PSUs.
Organic Revenue Growth Metrics
Organic revenue growth comprised the other 50% of the total PSU award granted to both Mr. Logan and Mr. Schopfer. The organic revenue growth performance period is three years, from January 1, 2022 through December 31, 2024. The following targets were set with respect to the organic revenue growth metric:
| | | | | | | | | | | | | | |
| Percentile | Payout |
| Below Threshold | | <5% | | 0% |
| Target | | ≥5% | | 100% |
Organic revenue growth is defined as reported revenue growth excluding the effects on revenue of currency translation and acquisitions. For acquisitions, revenue becomes organic upon the one-year anniversary of the completion of the
acquisition. For purposes of evaluating the organic revenue growth performance against the targets listed above, organic revenue growth is calculated as the compound annual organic revenue growth rate over the three-year measurement period. In no event will participants be eligible to receive more than 100% of the organic revenue growth PSUs.
Beginning in 2022, we anticipate that the Compensation Committee or the Board will consider approving equity incentive awards to our NEOs on an annual basis.
Prior to the Business Combination, Mr. Logan and Mr. Schopfer were granted profits interests which are summarized in more detail below under “Analysis of Fiscal 2021 Compensation _Compensation Elements _Pre-Business Combination Compensation.”
Other Elements of Compensation
Retirement Plans
We maintain a 401(k) retirement savings plan for our employees in the United States, including the NEOs, who satisfy certain eligibility requirements. The NEOs are eligible to participate in the 401(k) plan on the same terms as other full-time employees, including matching contributions which at the beginning of 2020 were equal to 100% of a participating employee’s contribution up to the first 1% of the employee’s eligible compensation and 50% of the employee’s contribution up to the next 5% of the employee’s eligible compensation. In 2021, we amended our matching contribution under the 401(k) plan such that matching contributions are currently equal to 100% of a participating employee’s contribution up to the first 2% of the employees’ eligible compensation and 50% of the employees’ contribution up to the next 4% of the employee’s eligible compensation.
Non-Qualified Deferred Compensation Plan
We maintain an unfunded, non-qualified deferred compensation plan (the “Deferred Compensation Plan”), in which Mr. Logan and one other managerial employee participate. Each year, participants can elect to defer up to 100% of their base salary, bonus, commission and/or short- and long-term incentive compensation, as applicable, under the Deferred Compensation Plan. We do not make any matching, discretionary or other similar contributions to the Deferred Compensation Plan on behalf of participants.
Participants may elect to receive compensation they have deferred upon certain qualifying distribution events (e.g., separation from service, death, disability or at a specified date) at which time account balances are distributed in cash either in a lump sum or annual installments as elected by the participant. If no election is made, account balances are distributed in a lump sum. Annual installments can be for up to five years if the qualifying distribution event is a specified date or 15 years if the qualifying distribution event is due to a separation from service. Account balances are distributed in a single lump sum to the participant’s beneficiary upon the participant’s death or disability. Participants may also elect to receive in a single lump sum, in the event of a separation from service within two years of a change in control of the Company, the entire vested portion of their account balance that was otherwise reserved for payment in installments following a separation from service or at a specified date.
Account balances under the Deferred Compensation Plan are credited with a deemed investment return (or credited with a deemed investment loss), determined as if the account was invested in one or more investment funds made available by the administrator. Participants elect the investment fund(s) in which accounts will be deemed invested. Participants may change their investment elections on a daily basis. The investment vehicle is determined by the administrator if the participant fails to make an investment election.
Employee Benefits and Perquisites
All of our full-time employees in the United States, including the NEOs, are eligible to participate in health and welfare plans, including medical, dental and vision benefits, medical and dependent care flexible spending accounts, short-term and long-term disability insurance and life insurance. Additionally, each of the NEOs is entitled to company-paid premiums for long-term care insurance. Pursuant to his employment agreement, Mr. Logan is also entitled to reimbursement for certain air travel and automobile expenses, as described further below.
In addition to the benefits set forth in his employment agreement, Mr. Logan also receives a $100 per month allowance for automobile maintenance. The NEOs are each also entitled to reimbursement for the costs of an annual physical examination and financial planning services. We believe the benefits described above are necessary and appropriate to provide a competitive compensation package to our employees, including the NEOs.
Pre-Business Combination Compensation
Profits Interests
On June 16, 2021 and in connection with the Business Combination, the Sponsor agreed to issue 3,200,000 membership interests to Mr. Logan and 700,000 membership interests to Mr. Schopfer (collectively, the “Profits Interests”), pursuant to which Messrs. Logan and Schopfer will have an indirect interest in the founder shares held by the Sponsor. The Profits Interests are subject to service and performance vesting conditions and do not fully vest until all of the applicable conditions are satisfied, including the achievement of specified share price conditions. The grant of the Profits Interests is intended to be a one-time grant by the Sponsor in recognition of Messrs. Logan and Schopfer’s efforts in connection with the Business Combination. In addition, the Profits Interests are subject to certain forfeiture conditions. See “Part III, Item 13. Certain Relationships and Related Transactions, and Director Independence—Profits Interests” for more information.
Exit Bonuses
In 2020, each of the NEOs entered into a letter agreement with Mirion Technologies (Global) Ltd., pursuant to which they are entitled to cash bonuses (the “Exit Bonuses”) in the event of an “Exit” (as such term is defined below), subject to the applicable NEO remaining actively employed with the Company in good standing through the date of such Exit. The Exit Bonus program was established to incentivize officers and key executives to continue to grow the business with an eye towards an eventual liquidity event, upon which the Exit Bonuses would vest and be paid. The amount of each NEO’s Exit Bonus is calculated (i) for Messrs. Logan and Freed, as the product of (x) the number of Class A ordinary shares of Mirion Technologies (Topco), Ltd. that were subscribed for or acquired by Mr. Logan or Mr. Freed, as applicable, at a price per share equal to $9.65 and that Mr. Logan or Mr. Freed, as applicable, holds immediately prior to the consummation of the applicable Exit event, multiplied by (y) $8.65 and (ii) for Mr. Schopfer, as the product of (x) the number of Class A ordinary shares of Mirion that were subscribed for or acquired by Mr. Schopfer at a price per share equal to $9.99 and that Mr. Schopfer holds immediately prior to the consummation of the applicable Exit event, multiplied by (y) $8.99. For purposes of the Exit Bonuses, “Exit” means the transfer of shares (whether through a single transaction or a series of transactions) as a result of which any person, or persons connected (as defined in Section 252 of the U.K. Companies Act) or acting in concert (as defined in the City Code on Takeovers and Mergers) with such person, holds more than 50% of the Class A and Class B ordinary shares of Mirion. The consummation of the Business Combination constituted an Exit, and the Exit Bonuses vested and became payable in connection with the closing of the Business Combination.
Employment Arrangements
Logan Employment Agreement
On August 15, 2006, the Company entered into an employment agreement with Mr. Logan, which was subsequently amended on December 22, 2008, January 1, 2009, June 16, 2010, January 1, 2011, July 1, 2011, June 16, 2021, August 13, 2021 and December 27, 2021 (as amended, the “Logan Employment Agreement”), providing for his employment as Chief Executive Officer of the Company. The Logan Employment Agreement provides that Mr. Logan is entitled to an annual base salary and is eligible for an annual incentive bonus based on the Company’s achievement of targets and milestones as determined by the Board. Beginning in calendar year 2022, Mr. Logan is also eligible to receive an annual long-term equity incentive grant having a target total grant date value equal to $2,700,000 (in 2022, 2/3 of this grant will consist of RSUs and 1/3 will consist of PSUs, in subsequent calendar years the mix of RSUs and PSUs may be different, if determined by the Board in its discretion). Mr. Logan is also entitled to reimbursement for the following expenses: (i) reimbursement for first class air travel expenses, (ii) the cost of an annual local executive physical examination up to $10,000 and (iii) the costs of annual financial planning services, up to $5,000 per year.
Pursuant to the Logan Employment Agreement, upon the termination of Mr. Logan’s employment with the Company without Cause or by Mr. Logan for Good Reason, subject to his execution and non-revocation of a general release of claims against the Company, Mr. Logan will be entitled, in addition to any accrued amounts, to (i) continuation of his annual base salary for twenty-four (24) months following the date of the termination of Mr. Logan’s employment (provided that, if Mr. Logan is terminated within twenty-four (24) months of a “change in control” (as such term is defined in the Incentive Plan) that occurs after January 1, 2022, Mr. Logan will receive two (2) times the sum of his base salary and target bonus), (ii) a pro rata portion of Mr. Logan’s annual incentive bonus for the fiscal year in which the termination of his employment occurs, payable at the same time as such payment would otherwise have been made to Mr. Logan had his employment not been terminated, and (iii) continuation of any health benefits provided by the Company to Mr. Logan and his dependents for eighteen (18) months.
The Logan Employment Agreement also provides that in the event of the termination of Mr. Logan’s employment with the Company as a result of his death or permanent disability, Mr. Logan or his estate, as applicable, will be entitled, in addition to any accrued amounts, to a pro-rata annual incentive bonus and continued health benefits for 12 months for Mr. Logan and/or his family.
In addition, the employment agreement amendment entered into by Mr. Logan in December 2021 eliminated his right to a tax gross-up with respect to excise taxes relating to Sections 280G and 4999 of the Code and replaced that provision with a provision that requires that any parachute payments be reduced to the largest amount that would result in no portion of the payments being subject to an excise tax, if such reduction provides Mr. Logan with the best net after-tax result.
“Cause” is defined in the Logan Employment Agreement generally as Mr. Logan’s (i) commission of or engagement in an act of fraud, embezzlement, sexual harassment, dishonesty or theft in connection with Mr. Logan’s duties for the Company or any of its subsidiaries, (ii) material breach of or default under the Logan Employment Agreement or Mr. Logan’s non-disclosure agreement with the Company or any similar agreement with the Company or any of its subsidiaries (which such breach or default, if reasonably capable of being cured, is not cured within two business days after written notice thereof is received by Mr. Logan, or, if reasonably capable of being cured but not within two business days, if Mr. Logan has not commenced cure in good faith within such two business day period and completed such cure as promptly as reasonably practicable thereafter), (iii) conviction of, or plea of nolo contendere with respect to, a felony, or (iv) engagement in an act of gross negligence or willful failure to perform his duties or responsibilities, including the failure to follow in any material respect a direction or written policy of the board of directors of the Company (which such breach or default, if reasonably capable of cure, is not cured within five business days after written notice thereof or, if reasonably capable of cure but not within five business days, if Mr. Logan has not commenced cure in good faith within such five business day period and completed such cure as promptly as reasonably practicable thereafter).
“Good Reason” is defined in the Logan Employment Agreement generally as any of the following, without Mr. Logan’s written consent: (i) a reduction in Mr. Logan’s base salary, a material reduction or discontinuation of any material incentive compensation or expense reimbursement plan or the taking of any action with the purpose of materially adversely affecting Mr. Logan’s participation in benefits under any fringe benefit provided to Mr. Logan (other than with respect to such actions taken by the Company (other than a reduction in Mr. Logan’s base salary) as part of an overall plan by the Company and made applicable to the same extent to all Company employees), (ii) a diminution in Mr. Logan’s title or position or a significant diminution in Mr. Logan’s authorities, duties or responsibilities with respect to the Company, (iii) the requirement by the Company that Mr. Logan be based in an office which is more than twenty-five (25) miles as compared to Mr. Logan’s daily commute, immediately prior to such relocation, or from his primary residence to his then principal place of employment, or (iv) any failure by the Company to comply with any material provision of the Logan Employment Agreement or any other material agreement between Mr. Logan and the Company. If Mr. Logan provides written notice of termination of his employment for Good Reason for any of the circumstances described above, the Company will have the opportunity to cure such circumstances within fifteen (15) days of receipt of such notice. If Mr. Logan does not deliver to the Company a notice of termination of his employment within ninety (90) days after Mr. Logan has knowledge that an event constituting Good Reason has occurred, such event will no longer constitute Good Reason.
In addition, pursuant to a Confidentiality and Intellectual Property Agreement attached as an exhibit to the Logan Employment Agreement, Mr. Logan is subject to a perpetual obligation not to disclose the confidential information of the Company.
Schopfer Employment Agreement
On May 1, 2020, the Company entered into the Third Amended and Restated Employment Agreement with Mr. Schopfer, which was subsequently amended on December 27, 2021 (as amended, the “Schopfer Employment Agreement”). The Schopfer Employment Agreement provides that Mr. Schopfer is entitled to an annual base salary and is eligible for an annual performance bonus based on personal and corporate performance goals as determined by the Company’s board of directors. Mr. Schopfer is also entitled to (i) an annual allowance of $5,000 to cover costs for any personal financial or tax advisory services retained in connection with any matter arising as a result of Mr. Schopfer holding shares of, or any other investment in, the Company and (ii) reimbursement for the cost of an annual physical examination up to $5,000.
Pursuant to the Schopfer Employment Agreement, upon the termination of Mr. Schopfer’s employment with the Company without Cause or by Mr. Schopfer for Good Reason, subject to his execution and non-revocation of
a general release of claims against the Company, Mr. Schopfer will be entitled, in addition to any accrued amounts, to (i) continuation of his annual base salary for twelve (12) months following the date of the termination of Mr. Schopfer’s employment (such twelve (12)-month period, the “Schopfer Severance Period”) (provided that, if Mr. Schopfer's employment is terminated without Cause or for Good Reason within twelve (12) months of a “change in control” (as such term is defined in the Incentive Plan) that occurs after January 1, 2022, Mr. Schopfer will receive an amount equal to one (1) times the sum of his base salary and target bonus), (ii) a pro rata portion of Mr. Schopfer’s annual incentive bonus for the fiscal year in which the termination of his employment occurs, payable at the same time as such payment would otherwise have been made to Mr. Schopfer had his employment not been terminated , and (iii) continued payment by the Company, for the Schopfer Severance Period or, if earlier, until the date on which Mr. Schopfer commences employment
with and becomes eligible for health care benefits from a new employer, of the premiums associated with group health continuation coverage premiums for Mr. Schopfer and his dependents under COBRA.
The Schopfer Employment Agreement also provides that in the event of the termination of Mr. Schopfer’s employment with the Company as a result of his death or permanent disability, Mr. Schopfer or his estate, as applicable, will be entitled, in addition to any accrued amounts, to a pro-rata annual incentive bonus.
For purposes of the Schopfer Employment Agreement, “Cause” is defined in a manner that is substantially similar to the definition of such term in the Logan Employment Agreement, and “Good Reason” is defined in a manner that is substantially similar to the definition of such term in the Freed Employment Agreement.
In addition, pursuant to a Confidentiality, Non-Interference and Intellectual Property Agreement attached as an exhibit to the Schopfer Employment Agreement, Mr. Schopfer is subject to (i) a covenant restricting him from interfering with the business of the Company by soliciting, diverting or enticing away any officer, employee or consultant of the Company or any of its subsidiaries to accept employment with a third party for a period of 12 months following his termination of employment with the Company for any reason, (ii) a covenant restricting him in perpetuity from using the confidential information of the Company to solicit, divert or entice away (A) any actual or prospective customer of the Company or any of its subsidiaries to become a customer of any third party that is engaged in any business or operations that were also engaged in by the Company during Mr. Schopfer’s employment with the Company or (B) any customer or supplier to cease doing business with the Company or any of its subsidiaries and (iii) a perpetual obligation not to disclose the confidential information of the Company.
Freed Employment Agreement
On July 16, 2016, the Company entered into an employment agreement with Mr. Freed, which was subsequently amended on December 27, 2021 (as amended, the “Freed Employment Agreement”). The Freed Employment Agreement provides that Mr. Freed is entitled to an annual base salary and is eligible for an annual performance bonus based on personal and corporate performance goals as determined by the Board. Mr. Freed is also entitled to (i) an annual allowance of $5,000 to cover costs for any personal financial or tax advisory services retained in connection with any matter arising as a result of Mr. Schopfer holding shares of, or any other investment in, the Company and reimbursement for the cost of an annual physical examination and (ii) the cost of an annual local executive physical examination up to $5,000.
Pursuant to the Freed Employment Agreement, upon the termination of Mr. Freed’s employment with the Company without Cause or by Mr. Freed for Good Reason, subject to his execution and non-revocation of a general release of claims against the Company, Mr. Freed will be entitled, in addition to any accrued amounts, to (i) continuation of his annual base salary for twelve (12) months following the date of the termination of Mr. Freed’s employment with the Company (such twelve (12)-month period, the “Freed Severance Period”) (provided that, if Mr. Freed is terminated within twelve (12) months of a “change in control” (as such term is defined in the Incentive Plan) that occurs after January 1, 2022, Mr. Freed will receive an amount equal to one (1) times the sum of his base salary and target bonus), (ii) a pro rata portion of Mr. Freed’s annual incentive bonus for the fiscal year in which the termination of his employment occurs, payable at the same time as such payment would otherwise have been made to Mr. Freed had his employment not been terminated, and (iii) continued payment by the Company for the Freed Severance Period or, if earlier, until the date on which Mr. Freed commences employment with and becomes eligible for health care benefits from a new employer, of the premiums associated with group health continuation coverage premiums for Mr. Freed and his dependents under COBRA.
The Freed Employment Agreement also provides that in the event of the termination of Mr. Freed’s employment with the Company as a result of his death or permanent disability, Mr. Freed or his estate, as applicable, will be entitled, in addition to any accrued amounts, to a pro-rata annual incentive bonus.
For purposes of the Freed Employment Agreement, “Cause” is defined in a manner that is substantially similar to the definition of such term in the Logan Employment Agreement. Under the Freed Employment Agreement, “Good Reason” is defined generally as any of the following, without Mr. Freed’s consent: (i) a material reduction in Mr. Freed’s base salary, (ii) a material diminution in Mr. Freed’s authorities, duties or responsibilities with respect to the Company, (iii) the requirement by the Company that Mr. Freed be based in an office which increases his commute by more than twenty-five (25) miles in relation to Mr. Freed’s then-principal place of employment, or (iv) any material breach by the Company of any material provision of the Freed Employment Agreement. If Mr. Freed provides written notice of termination of his employment for Good Reason for any of the circumstances described above, the Company will have the opportunity to cure such circumstances within thirty (30) days of receipt of such notice. If Mr. Freed does not deliver to the Company a notice of termination of his employment within thirty (30) days after Mr. Freed has knowledge that an event constituting Good Reason has occurred, such event will no longer constitute Good Reason.
In addition, pursuant to a Confidentiality, Non-Interference and Intellectual Property Agreement attached as an exhibit to the Freed Employment Agreement, Mr. Freed is subject to (i) non-competition restrictions with respect to certain competitors of the Company in certain geographical locations for a period of 12 months following his termination of employment with the Company for any reason, (ii) non-solicitation restrictions (with respect to certain employees and customers of the Company) for a period of 12 months following his termination of employment for any reason and (iii) a perpetual obligation not to disclose the confidential information of the Company; provided that, if the Company wishes to enforce the 12-month non-competition restriction, the Company must pay Mr. Freed additional consideration equal to 50% the sum of his base salary and his pro rata incentive bonus.
Other Compensation Governance Practices
Share Ownership Guidelines
The Compensation Committee believes that purchasing and holding the Company’s shares of common stock create an incentive to manage the Company prudently. The Compensation Committee has established minimum share ownership requirements for our NEOs and certain other employees. Our CEO is required to hold at least 5x his base salary, our CFO is required to hold at least 3x his base salary and our other NEO(s) are required to hold at least 1x their base salary. In addition, our non-employee directors are required to hold 3x their annual cash retainer. Shares owned outright, unvested restricted stock and RSUs and shares or share equivalent units underlying deferred fees paid to directors are included in the calculation of share ownership.
Participants have five years to achieve these guidelines and must retain all of their net shares received as a result of the vesting, payment or distribution of any restricted stock units, performance stock units or director deferred stock units until this requirement is met.
Clawback Policy
In November 2021, the Company adopted a Clawback Policy that provides that in the event the Company is required to prepare an accounting restatement due to the material noncompliance of the Company with any financial reporting requirement under the securities laws, the Board will require reimbursement to the Company of any performance-based award made to any executive officer of the Company where: (i) the payment was predicated upon achieving certain financial results that were subsequently the subject of a substantial restatement of Company financial statements filed with the SEC; (ii) the members of the Board who are considered “independent” for purposes of the listing standards of NYSE determine the officer engaged in intentional misconduct that caused or substantially caused the need for the accounting restatement; and (iii) a lower payment would have been made to such officer based upon the restated financial results.
In each such instance, the Company will, to the extent practicable, seek to recover from the executive officer the amount in excess of what would have been awarded based on the corrected performance measures.
Tax and Accounting Considerations
Deductibility of Executive Compensation
Section 162(m) of the IRC (Section 162(m)) generally imposes a $1 million cap on the federal income tax deduction for compensation paid to our “covered employees” during any fiscal year. While the Compensation Committee considers the deductibility of awards as one factor in determining executive compensation, the Compensation Committee also looks at other factors in making its decisions, and, in the exercise of its business judgment and in accordance with its compensation philosophy, the Compensation Committee retains the flexibility to award compensation even if the compensation is not deductible by us for tax purposes, and to modify compensation that was initially intended to be tax deductible if it determines such modifications are consistent with our business needs.
Accounting for Stock-Based Compensation
The Compensation Committee takes accounting considerations into account in designing compensation plans and arrangements for our NEOs and other employees. We follow ASC Topic 718 for our stock-based compensation awards which requires us to measure the compensation expense for all share-based payment awards based on the grant date “fair value” of these awards.
Compensation Committee Report
The Compensation Committee has reviewed and discussed this CD&A with management and, based on such review and discussions, the Compensation Committee recommended to the Board that the CD&A be included in the Company’s Annual Report on Form 10-K and Registration Statement on Form S-1.
Respectfully submitted,
Robert A. Cascella (chair)
Steven W. Etzel
John W. Kuo
TABLES
2021 Summary Compensation Table
The following table sets forth the annual and long-term compensation awarded to or paid to the NEOs for services rendered to the Company in all capacities during (i) the period beginning July 1, 2020 and ending June 30, 2021 (the "Prior Fiscal Year") and (ii) the period beginning July 1, 2021 and ending December 31, 2021.
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Name and Principal Position | | Period | | Salary ($)(1) | | Bonus(2) ($) | | Stock Awards ($)(3) | | Non-Equity Incentive Plan Compensation ($)(4) | | Change in Pension Value and Nonqualified Deferred Compensation Earnings ($) | | All Other Compensation ($)(5)(6) | | Total ($) |
Thomas Logan Chairman and Chief Executive Officer | | July 1, 2021-December 31, 2021 | | 325,763 | | | 511,574 | | | 5,509,532 | | | 174,736 | | | 314,771 | | | 46,200 | | | 6,882,576 | |
| | July 1, 2020-June 30, 2021 | | 632,262 | | | | | 19,240,000 | | | 511,410 | | | 166,716 | | | 72,025 | | | 20,770,468 | |
Brian Schopfer Chief Financial Officer | | July 1, 2021-December 31, 2021 | | 195,195 | | | 123,990 | | | 1,101,892 | | | 71,038 | | | | | 10,439 | | | 1,502,554 | |
| | July 1, 2020-June 30, 2021 | | 355,227 | | | | | 4,208,750 | | | 177,613 | | | | | 15,964 | | | 4,757,554 | |
Michael Freed Chief Operating Officer | | July 1, 2021-December 31, 2021 | | 213,263 | | | 432,500 | | | | | 72,300 | | | | | 8,557 | | | 726,620 | |
| | July 1, 2020-June 30, 2021 | | 398,851 | | | | | | | 199,455 | | | | | 12,167 | | | 610,473 | |
(1) Amounts reflect the base salary in effect for each named executive officer during the applicable time period. For additional information, see “Base Salaries” above.
(2) Amounts shown in this column for the Stub 2021 period reflect each NEO's Exit Bonus, as discussed above under "Exit Bonuses".
(2) This column reflects:
a.This column represents the aggregate grant date fair value computed in accordance with ASC Topic 718 for the RSUs and PSUs granted to Messrs. Logan and Schopfer in 2021. The grant date fair value of PSU awards was calculated based on the expected value of the possible outcomes of the performance conditions related to these awards in accordance with ASC Topic 718 (excluding the effects of estimated forfeitures).
b.The grant date value of a one-time grant of profits interests in the Sponsor, which was approved and granted by the Sponsor in recognition of Messrs. Logan and Schopfer’s efforts in connection with the Business Combination. As discussed above under “Profits Interests,” the Sponsor granted Messrs. Logan and Schopfer the award of profits interests on June 16, 2021 in connection with the signing of the Business Combination Agreement. The profits interests award provides for service and performance-vesting, with the award only vesting upon the achievement of specified share price conditions. The grant date fair value of the profits interests is based upon a valuation model using Monte Carlo simulations in accordance with ASC Topic 718.
(4) This column reflects (i) for the Stub 2021 period, amounts earned under the Stub 2021 Incentive Plan and (ii) for the 2021 fiscal year, amounts earned under the FY'21 Incentive Plan.
(5) Prior Fiscal Year Amounts reflect: (i) for Mr. Logan, a $21,312 cash payment in respect of accrued vacation days, a $14,400 automobile allowance, a company contribution of $12,495 to Mr. Logan’s account under Mirion's 401(k) plan, a $5,000 reimbursement for financial planning services, $3,500 to cover the costs of an annual physical examination, $13,135 in stipends paid to Mr. Logan for time spent flying his personal aircraft to business events plus corresponding reimbursement for fuel costs associated with such flights, $1,200 for continued automobile maintenance and $983 in Company-paid long-term care insurance premiums; (ii) for Mr. Schopfer, company contributions of $10,848 to Mr. Schopfer’s account under Mirion's 401(k) plan, $2,500 to cover the costs of an annual physical examination, a $1,500 reimbursement for financial planning services, $500 to Mr. Schopfer’s Mirion-sponsored health savings account and $616
in Company-paid long-term care insurance premiums and (iii) for Mr. Freed, a company contribution of $11,454 to Mr. Freed’s account under Mirion’s 401(k) plan and $713 in Company-paid long-term care insurance premiums.
(6) Amounts reflect: (i) for Mr. Logan, a $26,091 cash payment in respect of accrued vacation days, a $7,200 automobile allowance, a company contribution of $3,419 to Mr. Logan’s account under the Company’s 401(k) plan, $4,079 to cover the costs of an annual physical examination, $4,320 in stipends paid to Mr. Logan for time spent flying his personal aircraft to business events plus corresponding reimbursement for fuel costs associated with such flights, $600 for continued automobile maintenance and $491 in Company-paid long-term care insurance premiums; (ii) for Mr. Freed, a company contribution of $8,200 to Mr. Freed’s account under the Company’s 401(k) plan and $356 in Company-paid long-term care insurance premiums; and (iii) for Mr. Schopfer, company contributions of $7,381 to Mr. Schopfer’s account under the Company’s 401( a $— reimbursement for financial planning services, $250 to Mr. Schopfer’s Company-sponsored health savings account and $308 in Company-paid long-term care insurance premiums.
GRANTS OF PLAN-BASED AWARDS
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | Estimated Future Payouts Under Non-Equity Incentive Plan Awards | | Estimated Future Payouts Under Equity Incentive Plan Awards | | All Other Stock Awards: Number of Shares of Stock or Units (#) (i) | | Grant Date Fair Value of Stock and Option Awards (l) |
Name
(a) | | Grant Date
(b) | | Threshold ($) (c) | | Target ($)
(d) | | Maximum ($) (e) | | Threshold (#) (f) | | Target (#) (g) | | Maximum (#) (h) | | |
| Thomas Logan | | | | | | | | | | | | | | | | | | |
| FY 2021 Bonus | | | | 170,811 | | | 511,410 | | | 818,256 | | | | | | | | | | | |
| 2021 Stub Bonus | | | | 87,924 | | | 263,245 | | | 421,192 | | | | | | | | | | | |
| RSU | | 12/27/2021 | | | | | | | | | | | | | | — | | | 3,999,996 | |
| PSU | | 12/27/2021 | | 500,000 | | | 1,000,000 | | | 2,000,000 | | | 47,709 | | | 95,419 | | | 190,839 | | | | | 1,509,536 | |
| Brian Schopfer | | | | | | | | | | | | | | | | | | |
| FY 2021 Bonus | | | | 88,807 | | | 177,613 | | | 355,226 | | | | | | | | | | | |
| 2021 Stub Bonus | | | | 49,929 | | | 99,857 | | | 199,714 | | | | | | | | | | | |
| RSU | | 12/27/2021 | | | | | | | | | | | | | | — | | | 799,991 | |
| PSU | | 12/27/2021 | | 100,000 | | | 200,000 | | | 400,000 | | | 9,541 | | | 19,083 | | | 38,167 | | | | | 301,901 | |
| Michael Freed | | | | | | | | | | | | | | | | | | |
| FY 2021 Bonus | | | | 99,713 | | | 199,425 | | | 398,850 | | | | | | | | | | | |
| 2021 Stub Bonus | | | | 50,816 | | | 101,632 | | | 203,264 | | | | | | | | | | | |
OUTSTANDING EQUITY AWARDS AT FISCAL YEAR-END
The following table presents, for each of the named executive officers, information regarding outstanding RSUs,
PSUs and profits interests as of December 31, 2021.
| | | | | | | | | | | | | | | | | | | | | | | | | | |
| | Stock Awards(1) |
| Name (a) | | Number of Shares or Units of Stock That Have Not Vested (#) (b) | | Market Value of Shares or Units of Stock That Have Not Vested ($) (c)(2) | | Equity Incentive Plan Awards: Number of Unearned Shares, Units or Other Rights That Have Not Vested (#) (d) | | Equity Incentive Plan Awards: Market or Payout Value of Unearned Shares, Units or Other Rights That Have Not Vested ($)(2) (e) |
| Thomas Logan | | 381,679 (1) | | 4.00 | | | 143,130(3) | | 1,498,571 | |
| | | | | | 3,200,000 (4) | | 33,504,000 | |
| Brian Schopfer | | 76,335 (1) | | | | 28,625(3) | | 299,704 | |
| | | | | | 700,000(4) | | 7,329,000 | |
| Michael Freed | | - | | - | | - | | - |
(1) The RSUs vest in four substantially equal installments of one-fourth per year on the first, second, third and fourth anniversary of the grant date.
(2) Represents the market value of the shares underlying each of the outstanding equity awards, based on the closing price
of our Class A common stock on the New York Stock Exchange on December 31, 2021, which was $10.47.
(3) The amount reported for the PSUs is the threshold number of shares. The actual amount earned will be determined in 2025 based on the actual achievement of the performance goals, subject to continued employment through the determination of the achievement of the performance goals.
(4) Each of the profits interests must achieve both service-vesting and performance-vesting conditions in order to vest, with (i) 50% of the time-vesting portion being satisfied on each of the second and third anniversaries of the closing of the Business Combination (subject to continued employment on each vesting date) and (ii) the performance-vesting portion being satisfied with respect to (x) 25% of the profits interest grant, upon the value of our Class A common stock trading at $14 per share or more for a specified period of time prior to the fifth anniversary of the closing of the Business Combination and (y) 75% of the profits interests grant, upon the value of our Class A common stock trading at $16 per share or more for a specified period of time prior to the fifth anniversary of the closing of the Business Combination. If the performance-vesting conditions are not met prior to the fifth anniversary of the closing of the Business Combination, the profits interests are forfeited for no consideration. The service-vesting condition will be deemed to have been achieved on a change in control.
NONQUALIFIED DEFERRED COMPENSATION
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Name (a) | | Executive Contributions in Last fiscal year ($) (b) | | Registrant Contributions in Last fiscal year ($) (c) | | Aggregate Earnings in Last fiscal year ($) (d)(1) | | Aggregate Withdrawals/Distributions ($) (e) | | Aggregate Balance at Last FYE ($) (f) |
| Thomas Logan | | $ | — | | | $ | — | | | $ | 481,487 | | | $ | — | | | $ | 2,949,661 | |
| Brian Schopfer | | — | | | — | | | — | | | — | | | — | |
| Michael Freed | | — | | | — | | | — | | | — | | | — | |
(1) This includes aggregate earnings for the period beginning July 1, 2020 and ending on December 31, 2021.
We maintain an unfunded, non-qualified deferred compensation plan (the “Deferred Compensation Plan”). Mr. Logan is
the only NEO who participates. Each year, participants can elect to defer up to 100% of their base salary, bonus,
commission and/or short- and long-term incentive compensation, as applicable, under the Deferred Compensation Plan.
We do not make any matching, discretionary or other similar contributions to the Deferred Compensation Plan on behalf
of participants.
Account balances under the Deferred Compensation Plan are credited with a deemed investment return (or credited with a
deemed investment loss), determined as if the account was invested in one or more investment funds made available by the
administrator. Participants elect the investment fund(s) in which accounts will be deemed invested. Participants may
change their investment elections on a daily basis. The investment vehicle is determined by the administrator if the
participant fails to make an investment election.
Mr. Logan did not make any contributions to the Deferred Compensation Plan during the period beginning July 1, 2020
and ending December 31, 2021.
POTENTIAL PAYMENTS UPON TERMINATION OR CHANGE IN CONTROL
The table below sets forth the amounts of the payments and benefits that each NEO would have been entitled to receive upon a qualifying termination of employment by the Company and/or the occurrence of a change in control, in each case assuming the relevant event occurred on December 31, 2021.
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Name | | Benefit | | Termination Without Cause or Resignation for Good Reason Other than in Connection with a Change in Control | | Termination Without Cause or Resignation for Good Reason in Connection with a Change in Control (1) | | Death or Disability | | Retirement |
| Thomas Logan | | Cash severance | | $ | 963,362 | | | $ | 963,362 | | | $ | 263,362 | | | |
| | Accelerated Vesting of Equity Awards | | $999,047 (2) | | $5,994,263(3) | | — | | | $999,047 (2) |
| | Health Benefits | | 21,857 | | | 21,857 | | | 21,857 | | | |
| | Total | | $ | 1,984,266 | | | $ | 6,979,482 | | | $ | 285,219 | | | $ | 999,047 | |
| | | | | | | | | | |
| Brian Schopfer | | Cash Severance | | $ | 548,628 | | | $ | 548,628 | | | $ | 98,628 | | | |
| | Accelerated Vesting of Equity Awards | | — | | | $1,199,045(3) | | — | | | — | |
| | Health Benefits | | 8,142 | | | 8,142 | | | — | | | — | |
| | Total | | $ | 556,770 | | | $ | 1,755,815 | | | $ | 98,628 | | | $ | — | |
| | | | | | | | | | |
| Michael Freed | | Cash Severance | | $ | 513,769 | | | $ | 513,769 | | | $ | 101,631 | | | |
| | Accelerated Vesting of Equity Awards | | — | | | — | | | — | | | — | |
| | Health Benefits | | 31,226 | | | 31,226 | | | — | | | — | |
| | Total | | $ | 544,995 | | | $ | 544,995 | | | $ | 101,631 | | | $ | — | |
(1) Change in control-related severance payments to which the NEOs are entitled under their respective employment agreements are payable only in connection with a change in control that occurs after January 1, 2022. Accordingly, for purposes of this table, each NEO’s cash severance payments upon a termination without Cause or resignation for Good Reason are the same whether or not the triggering event is in connection with a change in control.
(2) Upon Mr. Logan’s termination without Cause or resignation for Good Reason or Mr. Logan’s retirement, as applicable, the number of Mr. Logan’s Bridge RSUs that were scheduled to vest (had Mr. Logan’s employment not terminated) during the 12-month period immediately following the termination date will become immediately vested.
(3) If Mr. Logan or Mr. Schopfer is terminated without Cause or resigns for Good Reason within a specified period of time following a change in control (for Mr. Logan, 24 months; for Mr. Schopfer, 12 months), all of their respective then unvested Bridge RSUs and Bridge PSUs will become immediately vested.
DIRECTOR COMPENSATION TABLE
The following table sets forth a summary of the compensation we paid to each non-employee member of our Board for the period beginning July 1, 2020 and ending December 31, 2021. During the Prior Fiscal Year, the only member of the Board to receive compensation was Mr. Kingsley, whose only compensation was the granting of profits interests by our Sponsor. Other than as set forth in the table and described more fully below, we did not pay any compensation to, make any equity awards or non-equity awards to, or pay any other compensation to any of the other non-employee members of our Board in fiscal year 2021. Mr. Logan serves as Chief Executive Officer and therefore does not receive any additional compensation for his service as a director.
| | | | | | | | | | | | | | | | | | | | | | | | | | |
| Name(1) | | Fees Earned or Paid in Cash ($)(1) | | Stock Awards ($)(2)(3) | | All Other Compensation ($) | | Total ($) |
| Kenneth C. Bockhorst | | 15,175 | | | 60,962 | | | — | | | 76,137 | |
| Robert Cascella | | 17,159 | | | 60,962 | | | — | | | 78,121 | |
| Steven W. Etzel | | 17,159 | | | 60,962 | | | — | | | 78,121 | |
| Lawrence D. Kingsley | | 15,175 | | | 32,526,962 | (4) | | — | | | 32,542,137 | |
| John W. Kuo | | 17,159 | | | 60,962 | | | — | | | 78,121 | |
| Jody A. Markopoulos | | 15,175 | | | 60,962 | | | — | | | 76,137 | |
| Jyothsna (Jo) Natauri | | — | | | — | | | — | | | — | |
| Christopher Warren | | — | | | — | | | — | | | — | |
(1) The amounts reported in this column represent the aggregate dollar amount of all fees earned or paid in cash to each non-employee director in fiscal year 2021 for their service as a director, including any annual retainer fees, committee and/or chair fees.
(2) The amounts shown in this column relate to the pro rata annual RSU grant made to certain non-employee directors, as further described below under the heading “Director Compensation.” For the RSUs, the amounts reported in this column represent the grant date fair value of RSUs calculated in accordance with the provisions of ASC Topic 718.
(3) As of December 31, 2021, the number of shares underlying outstanding restricted stock units held by each of our non-employee Directors were as follows:
| | | | | |
| Name | Aggregate Number of Shares Underlying Restricted Stock Units |
| Kenneth C. Bockhorst | 5,817 |
| Robert Cascella | 5,817 |
| Steven W. Etzel | 5,817 |
| Lawrence D. Kingsley | 5,817 |
| John W. Kuo | 5,817 |
| Jody A. Markopoulos | 5,817 |
| Jyothsna (Jo) Natauri | - |
| Christopher Warren | - |
(4) Value includes the grant date value of a one-time grant of profits interests in the Sponsor, which was approved and granted by the Sponsor in recognition of Mr. Kingsley’s efforts in connection with the Business Combination. As discussed above under “Executive Compensation—Profits Interests,” the Sponsor granted Mr. Kingsley the award of profits interests on June 16, 2021 in connection with the signing of the Business Combination Agreement. The profits interests award provides for service and performance-vesting, with the award only vesting upon the achievement of specified share price conditions. The grant date fair value of the profits interests is based upon a valuation model using Monte Carlo simulations
in accordance with ASC Topic 718. Mr. Kingsley also received a pro rata annual RSU grant with a grant date fair value of $60,962, consistent with all other non-employee directors.
Director Compensation
Prior to the closing of the Business Combination, our directors did not otherwise receive any additional compensation for their service in their capacity as directors except for the grant of profits interests to Mr. Kingsley as set forth above. In connection with the Business Combination, we implemented a new director compensation program (the “Director Compensation Program”). Pursuant to the Director Compensation Program, non-employee directors will receive the following cash compensation, paid quarterly in arrears, for their service as members of the Board and certain sub-committees thereof:
| | | | | |
| Position | Annual Retainer |
| Board Service | $76,500 |
| plus (as applicable): | |
| Audit Committee Chair | $10,000 |
| Compensation Committee Chair | $10,000 |
| Nominating/Governance Committee Chair | $10,000 |
In lieu of cash, non-employee directors may elect to receive full payment of their retainers in shares of our common stock
on a quarterly basis. Payment of retainers in a combination of cash and stock is not permitted.
In addition, non-employee directors will receive grants of equity awards under the Incentive Plan. Each year, the Board or Compensation Committee will provide each non-employee director who will continue to serve on the Board with a grant of restricted stock units (“RSUs”) with an approximate grant date fair market value of $93,500. These annual equity awards vest quarterly and will be fully vested on the first anniversary of the grant date, subject to the non-employee director’s continued service on the Board through each such vesting date. A non-employee director who is elected or appointed to the Board at any time other than at the annual stockholder meeting will, at the time of such election or appointment, receive an award of RSUs with a grant date fair market value equal to the product of $93,500 multiplied by a fraction (i) the numerator of which is equal to the number of days between the date of the director’s initial election or appointment to the Board and the date which is the first anniversary of the date of the most recent annual stockholder meeting occurring before the new non-employee director is elected or appointed to the Board, and (ii) the denominator of which is 365.
Each of Jyothsna (Jo) Natauri and Christopher Warren have agreed to waive compensation under the Director Compensation Program. The Director Compensation Program also provides that the Company will reimburse non-employee directors for their ordinary, necessary and reasonable out-of-pocket travel expenses to cover in-person attendance at and participation in Board meetings, in accordance with the Company’s applicable expense reimbursement policies and procedures as in effect from time to time.
ITEM 12. SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNER AND MANAGEMENT AND RELATED STOCKHOLDER MATTERS
The following table sets forth information known to us regarding the beneficial ownership of our common stock as of February 22, 2022 by:
•each person who is known by us to be the beneficial owner of more than 5% of the outstanding shares of the Class A common stock;
•each current executive officer and director of the Company; and
•all executive officers and directors as a group.
The information below is based on an aggregate of 199,523,292 shares of Class A common stock and 8,560,540 shares of Class B common stock issued and outstanding as of February 22, 2022. Beneficial ownership is determined according to the rules of the SEC, which generally provide that a person has beneficial ownership of a security if she, he or it possesses sole or shared voting or investment power over that security, including options and warrants that are currently exercisable or exercisable within 60 days. Unless otherwise indicated, the Company believes that all persons named in the table below have sole voting and investment power with respect to all shares of common stock beneficially owned by them:
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Name and Address of Beneficial Owners(1)(2) | | Number of Shares of Class A Common Stock6 | | Ownership
Percentage
of Class A
Common
Stock
(%) | | Number of
Shares of
Class B
Common
Stock | | Ownership
Percentage
of Class B
Common
Stock
(%) | | Ownership
Percentage
of Common
Stock
(%) |
| 5% Holders (Other than Directors and Executive Officers) | | | | | | | | | | |
GS Sponsor II LLC(3)(4) | | 24,525,000 | | 11.8 | % | | — | | — | | | 11.3 | % |
GSAM Holdings LLC(3)(4) | | 46,750,000 | | 22.5 | % | | — | | — | | | 21.6 | % |
GSAH II PIPE Investors Employee LP(5) | | 17,199,900 | | 8.6 | % | | — | | — | | | 8.3 | % |
Alyeska Investment Group, L.P. (6) | | 14,939,633 | | 7.5 | % | | — | | — | | | 7.1 | % |
Charterhouse Parties(7) | | 24,746,855 | | 12.4 | % | | — | | — | | | 11.9 | % |
| Directors and Executive Officers | | | | | | | | | | |
Thomas D. Logan(8) | | — | | — | | | 4,140,388 | | 48.4 | % | | 2.0 | % |
Lawrence D. Kingsley(9) | | 503,569 | | * | | 0 | | — | | | * |
Brian Schopfer(10) | | — | | — | | | 740,845 | | 8.7 | % | | * |
| Michael Freed | | — | | — | | | 935,818 | | 10.9 | % | | * |
Jyothsna (Jo) Natauri(11) | | — | | — | | | — | | | — | | | — | |
| Christopher Warren | | — | | — | | | — | | | — | | | — | |
Steven W. Etzel (12) | | 3,569 | | * | | — | | | — | | | * |
Kenneth C. Bockhorst (12) | | 3,569 | | * | | — | | | — | | | * |
Robert A. Cascella (12) | | 3,569 | | * | | — | | | — | | | * |
John W. Kuo (12) | | 3,569 | | * | | — | | | — | | | * |
Jody A. Markopoulos (12) | | 3,569 | | * | | — | | | — | | | * |
| All directors and executive officers as a group (11 individuals) | | 500,000 | | * | | 5,817,051 | | 68.0 | % | | 3.0 | % |
| | | | | |
| * | Less than one percent |
| (1) | Unless otherwise noted, the business address of each of the following entities or individuals is Mirion Technologies, Inc., 1218 Menlo Drive, Atlanta, Georgia 30318. |
| | | | | |
| (2) | The shares of our Class B common stock are paired, one-for-one, with shares of IntermediateCo Class B common stock. Such paired interests may be redeemed by the holder and, at our option, settled by a one-for-one exchange for shares of Class A common stock or a cash amount per share based on an average trailing stock price of Company Class A common stock. See “Certain Relationships and Related Transactions, and Director Independence—IntermediateCo Charter.” The founder shares are subject to certain vesting conditions upon a Founder Share Vesting Event. Holders of the founder shares are entitled to vote such founder shares and receive dividends and other distributions with respect to such founder shares prior to vesting, but such dividends and other distributions with respect to unvested founder shares will be set aside by the Company and shall only be paid to the holders of the founder shares upon the vesting of such founder shares. The founder shares will be forfeited to the Company for no consideration if they fail to vest on or before October 20, 2026. |
| (3) | GSAM Holdings LLC is the managing member of GS Sponsor II LLC. GSAM Holdings LLC is a wholly owned subsidiary of The Goldman Sachs Group, Inc. In addition to the shares held by GS Sponsor II LLC, GS Acquisition Holdings II Employee Participation LLC (“Participation LLC”) and GS Acquisition Holdings II Employee Participation 2 LLC (“Participation 2 LLC”), each of which is managed by a subsidiary of GSAM Holdings LLC, directly owns 1,325,000 founder shares and 1,400,000 founder shares, respectively. Each of GSAM Holdings LLC and The Goldman Sachs Group, Inc. may be deemed to beneficially own the shares held by GS Sponsor II LLC, Participation LLC and Participation 2 LLC by virtue of their direct and indirect ownership, as applicable, over GS Sponsor II LLC, Participation LLC and Participation 2 LLC. Each of GSAM Holdings LLC and The Goldman Sachs Group, Inc. disclaims beneficial ownership of any such shares except to the extent of their respective pecuniary interest therein. Further, each of GSAM Holdings LLC and The Goldman Sachs Group, Inc. may be deemed to beneficially own the shares held by the PIPE Participation LLCs (as defined below) but disclaims beneficial ownership of any such shares except to the extent of its pecuniary interest therein. |
| (4) | Interests shown for GS Sponsor II consist of (i) 16,025,000 founder shares and (ii) 8,500,000 shares of Class A common stock underlying the private placement warrants. Interests shown for GSAM Holdings consist of (i) 18,750,000 founder shares, (ii) 8,500,000 shares of Class A common stock underlying the private placement warrants and (iii) 19,500,000 shares of Class A common stock held by the PIPE Participation LLCs. |
| (5) | Each of GSAH II PIPE Investors Employee LP and NRD PIPE Investors LP (together the “PIPE Participation LLCs”) is a limited partnership controlled by its general partner and its investment manager, both of which are indirect wholly-owned subsidiaries of The Goldman Sachs Group, Inc. See the disclosure regarding Goldman Sachs under “Part III, Item 13. Certain Relationships and Related Transactions, and Director Independence —Related Party Payments” for information concerning certain relationships between Goldman Sachs and Mirion. Each limited partner of the PIPE Participation LLCs (including Jyothsna (Jo) Natauri, a Mirion director, and certain direct or indirect subsidiaries of The Goldman Sachs Groups, Inc.) will have the right to request that the applicable PIPE Participation LLC use its reasonable efforts to sell a portion of the registrable securities held by it under Mirion's registration statement on Form S-1 filed with and declared effective by the SEC on October 27, 2021 and November 2, 2021, respectively. The business address of each of the GS PIPE Participation LLCs is 200 West Street, New York, New York 10282. |
| (6) | Each of the Alyeska Investment Group, L.P., Alyeska Fund GP, LLC and Anand Parekh share voting and dispositive power with regard to shares of Class A common stock of the Company. The business address for each is 77 West Wacker Drive, 7th Floor, Chicago, IL 60601. Interests shown include 7,388,191 shares of Class A common stock issued to Alyeska and its affiliated entities in connection with the PIPE investment, 6,551,442 shares of publicly-traded common stock and 1,000,000 shares of Class A common stock underlying public warrants. |
| (7) | Represents (i) 13,233,013 shares of Class A common stock held by CCP IX LP No. 1; (ii) 11,028,610 shares of Class A common stock held by CCP IX LP No. 2; (iii) 363,920 shares of Class A common stock held by CCP IX Co-investment LP; and (iv) 121,312 shares of Class A common stock held by CCP IX Co-Investment No. 2 LP (together, “CCP IX”). Charterhouse General Partners (IX) Ltd (“CGP IX”) is the general partner of each of the limited partnerships comprising CCP IX. Charterhouse Capital Partners LLP (“CCP”) acts as the investment adviser to CGP IX. CCP’s advice with respect to investment decisions requires the approval of its Investment Committee comprised of 10 members, including the approval of CCP’s Managing Partner, which is currently Lionel Giacomotto. However, it is CGP IX which ultimately makes all investment decisions. As a result, CGP IX may be deemed to have beneficial ownership of the securities held by the limited partnerships comprising CCP IX. CGP IX is managed by a five member board of directors. Each of the CGP IX board members disclaims beneficial ownership of the securities beneficially owned by each of the limited partnerships comprising CCP IX, except to the extent of their pecuniary interest therein, if any. The address for each of the foregoing persons’ principal business office is 6th Floor, Belgrave House, 76 Buckingham Palace Road, London, SW1W 9TQ. |
| (8) | Mr. Logan’s shares consist of (i) 1,544,017 shares of Class B common stock held by Mr. Logan; (ii) 865,455 shares of Class B common stock held by the J.P. Morgan Trust Company of Delaware in its capacity as Trustee of the Mary Hancock Logan GST Exempt Trust; (iii) 865,455 shares of Class B common stock held by the J.P. Morgan Trust Company of Delaware in its capacity as Trustee of the Alison Paige Logan GST Exempt Trust; and (iv) 865,461 shares of Class B common stock held by the J.P. Morgan Trust Company of Delaware in its capacity as Trustee of the Thomas Darrell Logan, Jr. GST Exempt Trust. The J.P. Morgan Trust Company of Delaware in its capacity as Trustee of the foregoing trust entities has sole voting and dispositive power over the shares held by such trust entities; Mr. Logan disclaims beneficial ownership of any such shares except to the extent of his pecuniary interest therein. Mr. Logan’s shares exclude 3,200,000 shares of Class A common stock in which he has an interest due to his profits interests, which are subject to vesting requirements. See “Part III, Item 13. Certain Relationships and Related Transactions, and Director Independence—Profits Interests.” |
| (9) | Mr. Kingsley’s shares include (i) 3,569 shares of Class A common stock issuable pursuant to the vesting and settlement of RSUs held by Mr. Kingsley; (ii) 350,000 shares of Class A common stock held by the Diane Kingsley Revocable Trust and (iii) 150,000 shares held by the Lawrence D. Kingsley 2015 Family Irrevocable Trust, Mr. Kingsley’s shares exclude 4,200,000 shares of Class A common stock in which he has an interest due to his profits interests, which are subject to vesting requirements. See “Part III, Item 13. Certain Relationships and Related Transactions, and Director Independence—Profits Interests.” |
| (10) | Mr. Schopfer’s shares exclude 700,000 shares of Class A common stock in which he has an interest due to his profits interests, which are subject to vesting requirements. See “Part III, Item 13. Certain Relationships and Related Transactions, and Director Independence—Profits Interests.” |
| (11) | Ms. Natauri’s shares exclude 50,000 shares of Class A common stock held by GSAH II PIPE Investors Employee LP, and Ms. Natauri holds investment power over such shares. Voting decisions are made for the GSAH II Pipe Investors Employee LP by its investment manager, Goldman Sachs & Co. LLC, an affiliate of The Goldman Sachs Group, Inc. |
| (12) | Includes 3,569 shares of Class A common stock issuable pursuant to the vesting and settlement of RSU's |
ITEM 13. CERTAIN RELATIONSHIPS AND RELATED TRANSACTIONS, AND DIRECTOR INDEPENDENCE
IntermediateCo Charter
In connection with the Business Combination, we formed IntermediateCo as a direct subsidiary. IntermediateCo owns, directly or indirectly, all of our operating subsidiaries. As the holder of 100% of the voting securities of IntermediateCo, we have control over all of the affairs and decision making of IntermediateCo. As such, through our officers and directors, we are responsible for all operational and administrative decisions of IntermediateCo and the day-to-day management of IntermediateCo. We will fund any dividends to our stockholders by causing IntermediateCo to make distributions to us and the holders of IntermediateCo Class B common stock (including us) on a ratable basis.
Under the IntermediateCo Charter, the holders of IntermediateCo Class B common stock have the right (subject to the terms of our Charter), to require IntermediateCo to redeem all or a portion of their shares of IntermediateCo Class B common stock for, at our election, (1) newly issued shares of our Class A common stock on a one-for-one basis or (2) a cash payment equal to the product of the number of shares of IntermediateCo Class B common stock subject to redemption and the arithmetic average of the closing stock prices for a share of our Class A common stock for each of three (3) consecutive full trading days ending on and including the last full trading day immediately prior to the date of redemption (subject to customary adjustments, including for stock splits, stock dividends and reclassifications). If we decide to make a cash payment, the holder of IntermediateCo Class B common stock has the option to rescind its redemption request within a specified time period. The IntermediateCo Charter requires that we contribute, as applicable, cash or shares of our Class A common stock to IntermediateCo in exchange for an amount of newly-issued shares of IntermediateCo Class A common stock equal to the number of shares of IntermediateCo Class B common stock redeemed from the holders of IntermediateCo Class B common stock. IntermediateCo will then distribute the cash or shares of our Class A common stock to such holder of IntermediateCo Class B common stock to complete the redemption. In the event of a redemption request by a holder of IntermediateCo Class B common stock, we may, at our option, effect a direct exchange of cash or our Class A common stock for IntermediateCo Class B common stock in lieu of such a redemption. Shares of our Class B common stock will be canceled on a one-for-one basis if we, following a redemption request of a holder of IntermediateCo Class B common stock, redeem or exchange such holder’s IntermediateCo Class B common stock pursuant to the terms of the IntermediateCo Charter.
If at any time we issue a share of our Class A common stock or any other equity security with economic rights, the net proceeds received by us with respect to such share, if any, shall be concurrently contributed to IntermediateCo and IntermediateCo shall issue to us one share of IntermediateCo Class A common stock (or a corresponding other equity security of IntermediateCo), unless such share was issued by us solely to fund the purchase of a share of IntermediateCo Class B common stock from a holder of IntermediateCo Class B common stock (upon an election by us to exchange such IntermediateCo Class B common stock in lieu of redemption following a redemption request by such holder of IntermediateCo Class B common stock), in which case such net proceeds shall instead be transferred to the selling holder of IntermediateCo Class B common stock as consideration for such purchase, and IntermediateCo will not issue an additional share of Class A common stock to us. Similarly, (i) IntermediateCo may not issue any additional shares of its Class A common stock or Class B common stock to us or any of our subsidiaries unless substantially simultaneously therewith we or any of our subsidiaries issue or sell an equal number of shares of our Class A common stock, (ii) IntermediateCo may not issue any additional shares of its Class B common stock to any person other than us or any of our subsidiaries unless substantially simultaneously therewith we issue or sell an equal number of shares of our Class B common stock to such person and (iii) IntermediateCo may not issue any other equity securities to us or any of our subsidiaries unless substantially simultaneously therewith, we or such subsidiary issues or sells, to another person, an equal number of shares of a new class or series of equity securities of us or such subsidiary with substantially the same rights to dividends and distributions (including distributions upon liquidation) and other economic rights as those of such equity securities of IntermediateCo. Conversely, if at any time any shares of our Class A common stock are redeemed, purchased or otherwise acquired by us or any of our subsidiaries, IntermediateCo will substantially simultaneously therewith redeem, purchase or otherwise acquire an equal number of shares of its common stock held by us or our subsidiaries, upon the same terms and for the same price per security, as the shares of our Class A common stock are redeemed, purchased or otherwise acquired. In addition, IntermediateCo will not effect any subdivision (by any unit split, unit distribution, reclassification, reorganization, recapitalization or otherwise) or combination (by reverse unit split, reclassification, reorganization, recapitalization or otherwise) of its common stock unless it is accompanied by a substantively identical subdivision or combination, as applicable, of each class of our common stock, and we will not effect any subdivision or combination of any class of our common stock unless it is accompanied by a substantively identical subdivision or combination, as applicable, of the IntermediateCo common stock.
The IntermediateCo Charter provides that, in the event that a tender offer, share exchange offer, issuer bid, take-over bid, recapitalization or similar transaction with respect to our Class A common stock is proposed by us or to us and our stockholders and approved by our board of directors or is otherwise consented to or approved by our board of directors, the holders of paired interests comprised of shares of IntermediateCo Class B common stock and shares of our Class B common stock will be permitted to participate in such offer by delivery of a notice of redemption or exchange that is effective immediately prior to the consummation of such offer. In the case of any such offer proposed by us, we are obligated to use our commercially reasonable efforts to enable and permit the holders of such paired interests to participate in such offer to the same extent or on an
economically equivalent basis as the holders of shares of our Class A common stock without discrimination. In addition, we are obligated to use our reasonable efforts to ensure that the holders of such paired interests may participate in each such offer without being required to redeem or exchange IntermediateCo Class B common stock.
The IntermediateCo Charter provides that, except for transfers to us as provided above or to certain permitted transferees, the IntermediateCo Class B common stock may not be sold, transferred or otherwise disposed of.
Subject to certain exceptions, IntermediateCo will indemnify all of its directors and officers and other related parties, against all losses or expenses arising from claims or other legal proceedings in which such person (in its capacity as such) may be involved or become subject to in connection with IntermediateCo’s business or affairs or the IntermediateCo Charter or any related document.
Director Nomination Agreements
At the closing of the Business Combination (the “Closing”), we and CCP IX LP No. 1, CCP IX LP No. 2, CCP IX Co-Investment LP and CCP IX Co-Investment No. 2 LP (each acting by its general partner, Charterhouse General Partners (IX) Limited) (collectively, the “Charterhouse Parties” or the “Charterhouse Holders”) entered into a director nomination agreement (the “Charterhouse Director Nomination Agreement”) that provides the Charterhouse Parties with a right to representation on our Board. The Charterhouse Director Nomination Agreement grants the Charterhouse Parties the ongoing right (but not the obligation) to appoint or nominate to the Board of Directors one (1) individual (the “Charterhouse Director”), to serve as director of the Company. The Charterhouse Parties have designated Chris Warren as the initial Charterhouse Director. The Charterhouse Director Nomination Agreement will terminate automatically when the Charterhouse Parties, collectively with their respective affiliates, hold less than 5% of our then outstanding common stock, or upon the mutual written agreement of the parties.
At the Closing, we and the Sponsor also entered into a director nomination agreement (the “GS Director Nomination Agreement”) that provides the Sponsor with a right to representation on our Board. The GS Director Nomination Agreement grants the Sponsor the ongoing right (but not the obligation) to appoint or nominate to the Board of Directors two (2) individuals (the “GS Sponsor Directors”), to serve as director of the Company. The GS Sponsor has designated Larry Kingsley and Jo Natauri as the initial GS Sponsor Directors. The GS Director Nomination Agreement will terminate automatically when the Sponsor, GS Employee Participation and GS Employee Participation 2 (collectively, the "GS Holders"), collectively with their respective affiliates, hold less than 50% of the founder shares held by them at the Closing, or upon the mutual written agreement of the parties.
Amended and Restated Registration Rights Agreement
At the Closing, we entered into the Amended and Restated Registration Rights Agreement (the “RRA”) with the Sponsor, the GS Holders, GS II PIPE Investors Employee LP, NRD PIPE Investors LP, the Charterhouse Holders and all of the other pre-Business Combination shareholders of Mirion ( collectively, with each other person who has executed and delivered a joinder thereto, the “RRA Parties”) pursuant to which the RRA Parties are entitled to registration rights in respect of our Class A common stock held by the RRA Parties, or issuable upon redemption of shares of IntermediateCo Class B common stock or upon exercise of warrants to purchase shares of our Class A common stock held by them, in each case at the closing of the Business Combination (these securities are collectively referred to as the “Registrable Securities”). In addition, the Charterhouse Holders are entitled to registration rights on any outstanding shares of our common stock acquired by them following the Closing to the extent such securities are “restricted securities” or “control securities” within the meaning of Rule 144 under the Securities Act.
The RRA provides that we will use commercially reasonable efforts to file with the SEC a shelf registration statement registering the resale of certain shares of our Class A common stock and certain other equity securities of the Company held by the RRA Parties. We filed this shelf registration statement (the “Resale S-1”) on October 27, 2021 and it was declared effective on November 2, 2021. Each of (i) the Charterhouse Holders, (ii) the GS Holders or (iii) the holders of at least thirty percent (30%) in interest of the then outstanding registrable securities (each of (i), (ii) or (iii), the “Demanding Holders”) will be entitled to certain demand registration rights in connection with an underwritten offering. The Charterhouse Holders also have an exclusive right for a 90-day period beginning on April 19, 2022 (the “Charterhouse Demand Period”) to exercise a single demand right. The Demanding Holders are, at any time and from time to time on or after the date the Charterhouse Demand Period ends, entitled to demand registrations of all or part of their registrable securities. Such demand registrations are subject to certain offering thresholds, applicable lock-up restrictions and certain other conditions.
The Resale S-1 registered: (1) the issuance of up to 35,810,519 shares of Class A common stock upon (i) the exercise of warrants to purchase 27,249,979 shares of Class A common stock at an exercise price of $11.50 per share of Class A common stock, including the public warrants and the private placement warrants; and (ii) the redemption of 8,560,540 shares of IntermediateCo Class B common stock; and (2) the resale of up to 152,157,565 shares of Class A common stock consisting of (i) 116,347,025 shares of issued and outstanding shares of Class A common stock; (ii) 18,750,000 founder shares subject to vesting requirements; (iii) 8,500,000 shares of Class A common stock issuable upon the exercise of the private placement warrants; and
(iv) 8,560,540 shares of Class A common stock issuable upon the redemption of 8,560,540 shares of IntermediateCo Class B common stock.
Lockup Restrictions
Pursuant to the RRA, holders of any shares of our common stock or paired interests received by such holder as consideration pursuant to the Business Combination Agreement (such holders, the “Target Stockholders, and such common stock or paired interests, the “Lock-Up Securities”) will be subject to certain transfer restrictions described below (the “Lock-Up Restrictions”). Pursuant to the Lock-Up Restrictions, Target Stockholders may not (a) sell, assign, offer to sell, contract or agree to sell, hypothecate, pledge, grant any option to purchase or otherwise dispose of or agree to dispose of, directly or indirectly, or establish or increase a put equivalent position or liquidation with respect to or decrease a call equivalent position within the meaning of Section 16 of the Exchange Act with respect to, any security, (b) enter into any hedging, swap or other arrangement that transfers to another, in whole or in part, any of the economic consequences of ownership of any security, whether any such transaction is to be settled by delivery of such securities, in cash or otherwise, or (c) publicly announce any intention to effect any transaction specified in clause (a) or (b) (each of clauses (a), (b) and (c), a “Transfer”), through and including April 18, 2022 (the “Lock-Up Period”).
Notwithstanding the foregoing, the Lock-Up Securities may be transferred during the Lock-Up Period:
(i)by will, other testamentary document or intestacy;
(ii)as a bona fide gift or gifts, including to charitable organizations or for bona fide estate planning purposes;
(iii)to any trust for the direct or indirect benefit of the Target Stockholder or the immediate family of the Target Stockholder, or if the Target Stockholder is a trust, to a trustor or beneficiary of the trust or to the estate of a beneficiary of such trust;
(iv)to a partnership, limited liability company or other entity of which such Target Stockholder and the immediate family of such Target Stockholder are the legal and beneficial owner of all of the outstanding equity securities or similar interests;
(v)if the Target Stockholder is a corporation, partnership, limited liability company, trust or other business entity, (A) to another corporation, partnership, limited liability company, trust or other business entity that is an affiliate (as defined in Rule 405 promulgated under the Securities Act) of such Target Stockholder, or to any investment fund or other entity controlling, controlled by, managing or managed by or under common control with such Target Stockholder or affiliates of such Target Stockholder (including, for the avoidance of doubt, where such Target Stockholder is a partnership, to its general partner or a successor partnership or fund, or any other funds managed by such partnership), or (B) as part of a distribution to members or stockholders of such Target Stockholder;
(vi)to a nominee or custodian of any person or entity to whom a Transfer would be permissible under clauses (i) through (v) above;
(vii)in the case of an individual, by operation of law, such as pursuant to a qualified domestic order, divorce settlement, divorce decree, separation agreement or related court order;
(viii)with the prior written consent of the Board (subject to the determination of the Board in its sole discretion at any time); provided such consent must be approved by each of the Charterhouse Director (unless waived by the Charterhouse Holders) and the GS Directors (unless waived by the GS Sponsor Member);
(ix)from an employee or a director of, or a service provider to, us or any of our subsidiaries upon the death, disability or termination of employment or services, in each case, of such person; and
(x)pursuant to a bona fide third-party tender offer, merger, consolidation or other similar transaction that is approved by the Board and made to all holders of shares of the Mirion’s capital stock involving a Change of Control (as defined below) (including negotiating and entering into an agreement providing for any such transaction), provided that in the event that such tender offer, merger, consolidation or other similar transaction is not completed, the Target Stockholder’s Lock-Up Securities shall remain subject to the Lock-Up Restrictions.
provided that (x) in the case of any Transfer of Lock-Up Securities pursuant to clauses (i) through (vi), (1) such Transfer shall not involve a disposition for value; (2) the Lock-Up Securities shall remain subject to the Lock-Up Restrictions and the transferee shall sign a joinder to the Amended and Restated Registration Rights Agreement before such Transfer is effective; (3) any required public report or filing (including filings under Section 16(a) of the Exchange Act), shall disclose the nature of such Transfer and that the Lock-Up Securities remain subject to the Lock-Up Restrictions; and (4) there shall be no voluntary public disclosure or other announcement of such Transfer; and (y) a Target Stockholder may enter into a trading plan established in accordance with Rule 10b5-1 under the Exchange Act during the Lock-Up Period so long as no Transfers are effected under such trading plan prior to the expiration of the Lock-Up Period and no voluntary public disclosure or announcement of such plan is made.
In addition, pursuant to the RRA, during the Lock-Up Period and during the Charterhouse Demand Period (together, the “Charterhouse Demand Lock-Up Period”), (a) the GS Founder Share Members will not be permitted to transfer any shares of our common stock (other than to their permitted transferees) or request a demand registration and (b) GSAM Holdings LLC and its affiliates may not transfer any PIPE Shares (other than any such shares distributed to permitted transferees, including the GS
PIPE Participation LLCs) or request a demand registration (in each case of clauses (a) and (b), whether as part of a shelf registration, an unregistered transaction or otherwise); provided, however, that the Charterhouse Demand Lock-Up Period shall be extended for any day during which the Resale S-1 is not effective or sales pursuant to the Resale S-1 are suspended; provided, further, that the GS PIPE Participation LLCs (or their permitted transferees) shall also not request a demand registration during the Charterhouse Demand Lock-up Period.
Founder Shares
In July 2018, the Sponsor purchased an aggregate of 575 founder shares for an aggregate purchase price of $5,000. In April 2020 and June 2020, GSAH conducted stock splits, resulting in the Sponsor holding 20,125,000 founder shares, resulting in an effective purchase price per founder share of approximately $0.0003. The number of founder shares issued in the stock split was determined based on the expectation that the founder shares would represent 20% of the outstanding shares of common stock upon the completion of GSAH's initial public offering. Prior to the initial investment in GSAH of $5,000 by the Sponsor, GSAH had no assets, tangible or intangible. Following the partial exercise of the option to purchase additional shares, 1,375,000 founder shares were forfeited by the Sponsor on August 13, 2020, at no cost in order to maintain the number of founder shares equal to 20% of the outstanding shares of common stock, upon the completion of GSAH's initial public offering. In connection with the Closing, the founder shares became subject to certain vesting and forfeiture conditions. See “Part 1, Item 1. Business—Business Combination Overview.”
Private Placement Warrants
In connection with the completion of GSAH’s initial public offering, the Sponsor purchased an aggregate of 8,500,000 private placement warrants, each exercisable to purchase one share of the GSAH’s Class A common stock for $11.50 per share, at a price of $2.00 per private placement warrant, generating proceeds, before expenses, of $17,000,000. The private placement warrants (including the Class A common stock issuable upon exercise of the private placement warrants) are not redeemable by us so long as they are held by the Sponsor or its permitted transferees. Effective March 30, 2021, the Sponsor agreed not to transfer its private placement warrants.
Related Party Notes
On April 17, 2020, an affiliate of the Sponsor agreed to loan GSAH an aggregate amount of up to $300,000 to be used to pay a portion of the expenses related to GSAH's initial public offering pursuant to a promissory note (the “Note”). The Note was non-interest bearing, unsecured and payable on the earlier of December 31, 2020 and the closing of GSAH's initial public offering. On May 28, 2020, GSAH borrowed $300,000 under the Note. On July 2, 2020, the full $300,000 balance of the Note was repaid.
On November 12, 2020, the Sponsor agreed to loan GSAH up to an aggregate of $2,000,000 pursuant to the working capital note (the “Working Capital Note”). Any amounts borrowed under the Working Capital Note were non-interest bearing, unsecured and were due at the earlier of the date GSAH was required to complete its initial business combination pursuant to its amended and restated certificate of incorporation, as amended from time to time, and the closing of the initial business combination. On March 12, 2021, GSAH borrowed $1,500,000 under the Working Capital Note. On October 20, 2021, the $1,500,000 borrowed under the Working Capital Note was forgiven.
Sponsor Commitment
On March 11, 2019, the Sponsor provided GSAH with a commitment pursuant to which the Sponsor agreed that, if funds are needed by GSAH through June 12, 2020 to pay ordinary course expenses, the Sponsor would provide GSAH with liquidity of up to an aggregate of $2.0 million. The Sponsor will not receive any additional interest in GSAH in exchange for any such contribution and any liquidity provided under the commitment will be in the form of a contribution with respect to the Sponsor’s founder shares. This commitment was not exercised.
Administrative Services Agreement
GSAH entered into an agreement to pay an affiliate of the Sponsor a total of $10,000 per month for office space, utilities, administrative and support services. This agreement terminated upon the completion of the initial business combination. GSAH ceased paying these monthly fees as of October 20, 2021.
Subscription Agreements
Concurrently with the execution of the Business Combination Agreement, GSAH entered into a Subscription Agreement with GSAM Holdings, pursuant to, and on the terms and subject to the conditions of which, GSAM subscribed for 20,000,000 PIPE Shares of our Class A common stock for an aggregate purchase price equal to $200,000,000, subject to GSAM’s rights to syndicate prior to the Closing. The PIPE Investment was consummated substantially concurrently with Closing. GSAM
Holdings syndicated 17,199,900 and 2,300,100 shares of our Class A common stock from its PIPE investment to GSAH II PIPE Investors Employee LP and NRD PIPE Investors LP, respectively. GSAM Holdings also syndicated 500,000 shares of our Class A common stock from its PIPE investment to entities affiliated with Lawrence D. Kingsley, Chairman of the Board, at a price of $10.00 per share.
A&R Sponsor Agreement
In connection with the execution of the Business Combination Agreement, GSAH amended and restated that certain letter agreement, dated June 29, 2020 (the "Letter Agreement"), by and among GSAH, the Sponsor, GSAM Holdings, GS Employee Participation (collectively, the “Insiders”), pursuant to which, among other things, the Insiders agreed (i) to vote any shares of GSAH’s securities in favor of the Business Combination and other Business Combination proposals, (ii) not to redeem any shares of the Company’s Class A common stock or the Company’s Class B common stock, in connection with the optional stockholder redemption, and (iii) certain transfer restrictions. On October 20, 2021, the GSAH again amended and restated the Letter Agreement pursuant to which GS Employee Participation 2 became a party to the agreement.
Related Party Payments
Goldman Sachs & Co. LLC (“Goldman Sachs”), an affiliate of GSAH and the Sponsor (Thomas R. Knott, Chief Executive Officer, Chief Financial Officer and Secretary and a director of GSAH, is a Managing Director of Goldman Sachs and Raanan A. Agus, one of GSAH’s directors, is a Participating Managing Director of Goldman Sachs), acted as financial advisor to GSAH in connection with, and participated in certain of the negotiations leading to, the transaction contemplated by the Business Combination Agreement.
In connection with the Business Combination, an aggregate amount of approximately $33 million in deferred underwriting discount, advisory fees and placement agent fees, was paid to Goldman Sachs & Co. LLC in connection with the Closing of the Business Combination. Goldman Sachs & Co. LLC has provided certain financial advisory and/or underwriting services to GSAH from time to time for which the Investment Banking Division of Goldman Sachs has received, and may receive, compensation, including having acted as a joint bookrunner with respect to GSAH's initial public offering. Goldman Sachs also received a committed financing fee of $18,400,000 in connection with the financing of certain credit facilities.
In addition, Goldman Sachs (together with its affiliates) is a full service financial institution engaged in various activities, which may include sales and trading, commercial and investment banking, advisory, investment management, investment research, principal investing, hedging, market making, brokerage and other financial and non-financial activities and services. From time to time, Goldman Sachs and its affiliates have provided various investment banking and other commercial dealings unrelated to the Business Combination or the PIPE Investment to Mirion, the Charterhouse Parties and their affiliates, and GSAH and its affiliates, and has received customary compensation in connection therewith. In addition, Goldman Sachs and its affiliates may provide investment banking and other commercial services to us and our affiliates and to the Charterhouse Parties and their affiliates in the future, for which Goldman Sachs and its affiliates would expect to receive customary compensation. In the ordinary course of its business activities, Goldman Sachs and its affiliates, officers, directors and employees may make or hold a broad array of investments and actively trade debt and equity securities (or related derivative securities) and financial instruments (including bank loans) for their own accounts and for the accounts of their customers. Such investments and securities activities may involve securities and/or instruments of the Mirion, or its respective affiliates. Goldman Sachs and its affiliates may also make investment recommendations and/or publish or express independent research views in respect of such securities or financial instruments and may hold, or recommend to clients that they acquire, long and/or short positions in such securities and instruments.
Shareholder Notes
Mirion Technologies (HoldingSub1), Ltd. ("UKTopco") issued certain shareholder notes (the "Shareholder Notes"), to certain of its shareholders and members of Mirion management. All outstanding principal was due on March 31, 2026. The Shareholder Notes accrued payment-in-kind ("PIK") interest daily at a rate of 11.5% annually (other than a $70 million tranche that accrued interest at a rate of 6.0% annually until October 1, 2021 and then accrued interest at a rate of 11.5% annually thereafter) with such annual amount added to the outstanding principal amount on December 31 of each year in arrears. The Shareholder Notes could be prepaid without penalty at UKTopco’s option and are subordinate in right of payment to any indebtedness of UKTopco subsidiaries to banks or to other financial institutions (either currently existing or to occur in the future). The Shareholder Notes had a redemption right at the option of UKTopco by paying to the noteholder the full outstanding principal amount together with accrued but unpaid interest up to but excluding the redemption date. All of the Shareholder Notes were acquired by GSAH at the Closing for a price equal to the full outstanding principal amount together with all accrued but unpaid interest up to but excluding the Closing Date using a portion of the Business Combination consideration. As part of the Closing, GSAH contributed the Shareholder Notes to Mirion Topco and then the Shareholder Notes were extinguished in full.
Management Notes
UKTopco also issued certain notes (the "Management Notes"), to Thomas D. Logan, Mirion’s Chairman and Chief Executive Officer. The terms of the Management Notes were substantially similar to the Shareholder Notes, except that the Management Notes accrued payment-in-kind (PIK) interest daily at a rate of 11.5% annually with half of such annual amount added to the outstanding principal amount on December 31 of each year in arrears while the remaining half is payable in cash on December 31 of each year. At June 30, 2021 and 2020, there were $3.7 million and $3.4 million in Management Notes outstanding. All of the Management Notes were acquired by GSAH at the Closing for a price equal to the full outstanding principal amount together with all accrued but unpaid interest up to but excluding the Closing Date using a portion of the Business Combination consideration. As part of the Closing, GSAH contributed the Management Notes to Mirion Topco and then the Management Notes were extinguished in full.
Investment Agreement and Co-Investment Agreement
In November 2014, Mirion entered into an investment agreement (the “Investment Agreement”), with certain holders of our Management Notes, stockholder Notes, A Ordinary Shares and B Ordinary Shares, including certain members of the Mirion management team (the “Managers”), certain investors affiliated with the Charterhouse Holders (the “Investors”) and certain of Mirion’s subsidiaries. The Managers and the Investors are collectively referred to as the Ordinary Shareholders. The Investment Agreement provides the Ordinary Shareholders with certain registration rights, including the right to demand that Mirion file a registration statement and certain piggyback rights with respect to including their shares part of a registration statement that Mirion would otherwise file. The Investment Agreement also provides the Investors with certain information rights. The Investment Agreement also provides our Ordinary Shareholders with a right of first refusal with regard to certain issuances of our equity securities, which will not apply to, and will terminate upon, the consummation of the Business Combination. Finally, a majority of the Investors may from time to time appoint to, and remove from, the board of directors three non-executive directors; however, this board designation right will terminate upon the consummation of the Business Combination.
In June 2016, we entered into an amended and restated co-investment agreement (the “Co-Investment Agreement”), with the Managers, the Investors, additional investors (the “Co-Investors”) and certain of our subsidiaries, including Mirion Technologies (US), Inc., in order to finance the acquisition of Canberra Industries, Inc. and Canberra France. The terms of the Co-Investment Agreement are substantially similar to those of the Investment Agreement, however, there is no board designation right under the Co-Investment Agreement.
The Investment Agreement and the Co-Investment Agreement were terminated as of the Closing.
Director and Executive Officer Compensation
Please see “Part III, Item 11. Executive Compensation” for information regarding the compensation of Mirion’s directors and executive officers.
Employee Agreements
Mirion has entered into employment agreements with Mirion’s executive officers. For more information regarding these agreements, see “Part III, Item 11. Executive Compensation.”
Indemnification Agreements and Directors’ and Officers’ Liability Insurance
We have entered into indemnification agreements with certain of our former directors and executive officers. We also held an insurance policy that insures certain of our former directors and officers against certain liabilities. Effective upon the consummation of the Business Combination, we entered into new indemnification agreements with each of our directors and certain of our executive officers. We also obtained a new insurance policy that insures each of our directors and certain of our executive officers against certain liabilities.
Profits Interests
On June 16, 2021 and in connection with the Business Combination, the Sponsor issued 3,200,000 membership interests to Thomas Logan, 700,000 membership interests to Brian Schopfer and 4,200,000 membership interests to Lawrence Kingsley (collectively, the “Profits Interests”). The Profits Interests are intended to be treated as profits interests for U.S. income tax purposes, pursuant to which Messrs. Logan, Schopfer and Kingsley (the “Award Holders”) will have an indirect interest in the founder shares held by the Sponsor. The Profits Interests are subject to service and performance vesting conditions as described in this section, including the occurrence of the Closing, and do not fully vest until all of the applicable conditions are satisfied. In addition, the Profits Interests are subject to certain forfeiture conditions.
Fifty percent (50%) of the Profits Interests granted to each of Messrs. Logan and Schopfer service-vest on each of the second and third anniversaries of the Closing, and fifty percent (50%) of the Profits Interests granted to Mr. Kingsley service-vest on each of the first and second anniversaries of the Closing. All of the Profits Interests immediately service vest upon a change in control of GSAH, Mirion or any of their respective subsidiaries. If Messrs. Logan or Schopfer’s service (i) is terminated without “cause” or (ii) voluntarily ceases for “good reason” or his service terminates due to death or disability, 1/3, 2/3 and 100% respectively of the Profits Interests will become service-vested if the termination occurs before the respective first, second or third anniversaries of the Closing, and if Mr. Kingsley’s service terminates due to death or disability, 1/3 and 100% of the Profits Interests will become service-vested if the termination occurs before the respective first or second anniversary of the Closing. If Messrs. Logan or Schopfer voluntarily ceases to provide services without good reason after the second anniversaries of the Closing. In addition, if a change in control of GSAH, Mirion or any of their respective subsidiaries occurs within six months immediately following such termination of employment, then the all of the outstanding Profits Interests will service-vest as of immediately prior to the change of control. and if Mr. Kingsley voluntarily ceases to provide services after the first anniversary of the Closing, an additional of each such Award Holder’s Profits Interests will service-vest in respect of each full quarter the Award Holder provided services since the most recent service-vesting date.
Twenty-five (25%) and seventy-five (75%) of Profits Interests granted to each of Messrs. Logan and Schopfer become vested with respect to the performance vesting condition on the first trading day after the Closing for which the volume weighted average price of GSAH Class A common stock is $14.00 or $16.00, respectively, for at least 20 of 30 consecutive trading days, if such date occurs on or before the fifth anniversary of the Closing, and seventy-five (75%) and twenty-five (25%) of Profits Interests granted to Mr. Kingsley performance-vest on the first trading day after the Closing for which the volume weighted average price of GSAH Class A common stock is $12.00 or $14.00, respectively, for at least 20 of 30 consecutive trading days, if such date occurs on or before the fifth anniversary of the Closing. In addition, if a change in control of GSAH, Mirion or any of their respective subsidiaries occurs at any time on or prior to the fifth anniversary of the Closing and the per share value received is at least equal to the specified price then the performance vesting conditions of the applicable Profits Interests will be deemed satisfied.
The Profits Interests granted to an Award Holder are forfeited entirely if (i) the Award Holder’s services terminate for cause, (ii) Messrs. Logan or Schopfer voluntarily ceases to provide services without “good reason” prior to the second anniversary of the Closing or Mr. Kingsley voluntarily ceases to provide services prior to the first anniversary of the Closing, (iii) an Award Holder voluntarily ceases to provide services where grounds for a termination for cause exist, (iv) the Business Combination does not close prior to November 30, 2021 (or such later date as mutually agreed by Mirion and GSAH) or (v) an Award Holder materially breaches a restrictive covenant agreement. In addition, 320,000, 70,000 and 420,000 of the Profits Interests issued to each of Messrs. Logan, Schopfer and Kingsley, respectively, are forfeited from the total number of Profits Interests granted to each Award Holder that fully vest (if any) if Mirion fails to implement the compliance work plan and remedy any such failures.
Executive Loans
Mirion extended loans pursuant to individualized loan agreements, each dated as of June 2, 2020, with each of (i) Thomas Logan, with a principal amount of $528,005.98, (ii) Michael Freed, with a principal amount of $529,021.51 and (iii) Brian Schopfer, with an aggregate principal amount of $474,003.98 (collectively, the “Executive Loans”), each of which were intended to enable the applicable executive to acquire Class A ordinary shares of Mirion (the terms and circumstances of Mr. Schopfer’s Executive Loan are described further below). Each of the Executive Loans carries interest at a rate of 0.58% per annum. The Executive Loans became repayable, together with any accrued but unpaid interest thereon, on the Closing Date. The Executive Loan agreements contemplate repayment by the executives by applying (i) 50% of any after-tax amount of the applicable executive’s annual cash bonus in respect of any given year and (ii) any cash proceeds received by the executive directly attributable to his transfer of any Class A ordinary shares of Mirion, which such amounts each executive has expressly agreed may be deducted by Mirion to discharge the applicable Executive Loan.
Mr. Schopfer’s Executive Loan agreement provides for two separate tranches of loans. The first tranche amends and restates the terms applicable to a loan that Mirion originally extended to Mr. Schopfer on May 16, 2019, which, as of June 2, 2020, had $173,047.84, comprising principal and accrued but unpaid interest, outstanding thereunder. The second tranche of Mr. Schopfer’s Executive Loan consists of a principal amount of $168,313.50, applied as consideration for Mr. Schopfer’s acquisition of 110,000 shares of Mirion Class A common stock Mirion Topco and $55,681.96 principal amount of loan notes, plus interest.
Mirion also extended a loan to Mr. Schopfer on September 24, 2019 to enable Mr. Schopfer to repay a sign-on bonus granted to him by a previous employer that became repayable when Mr. Schopfer resigned his employment with such former employer to commence employment with Mirion (the “Schopfer Sign-On Bonus Loan”), the terms of which were amended and restated pursuant to an amended loan agreement with Mirion executed on June 2, 2020. On June 2, 2020, $132,642.64 of the Schopfer Sign-On Bonus Loan, comprising principal and accrued but unpaid interest, was outstanding. The terms of the Schopfer Sign-On Bonus Loan are substantially similar to those of Mr. Schopfer’s Executive Loan, including that the Schopfer Sign-On Bonus Loan carries interest at a rate of 0.58% per annum and became repayable on the Closing Date.
Each of the Executive Loans was repaid on October 20, 2021. Rights to receive an aggregate of 9,836 and 27,708 shares of Common Stock were surrendered to satisfy the Executive Loans of (i) Michael Freed having an aggregate principal amount of $98,368 and (ii) Brian Schopfer having an aggregate principal amount of $277,083, respectively.
Exit Bonuses
Please see “Part III, Item 11. Executive Compensation—Exit Bonuses” for information regarding cash bonuses that may have become payable to Mirion’s executive officers upon the Closing.
Management Fees
We were subject to agreements with certain of the Charterhouse Parties which obligated us to pay quarterly management fees of $0.1 million per year since 2015. In return, the Charterhouse Parties provided various investment banking services relating to financing arrangements, mergers and acquisitions and other services. During the Predecessor Stub Period and the fiscal years ended June 30, 2021, and June 30, 2020 and June 30, 2019, we paid the Charterhouse Parties an aggregate of $0.1 million, $0.1 million, $0.3 million and $0.2 million, respectively, for professional fees and expense reimbursements. Upon the completion of the Business Combination, these agreements with the Charterhouse Parties terminated and, as of December 31, 2021 we do not owe any additional payments for professional fees or have any expense reimbursements payable to the Charterhouse Parties.
Related Party Policy
Our Board of Directors adopted a written related party transaction policy that sets forth the following policies and procedures for the review and approval or ratification of related party transactions. A “related party transaction” is a transaction, arrangement or relationship in which the Company or any of its subsidiaries was, is, or will be a participant, the amount of which involved exceeds $120,000, and in which any related party had, has or will have a direct or indirect material interest. A “related party” means:
•any director (which term includes any director nominee of the Company) or executive officer of the Company;
•any immediate family member of any of the foregoing persons, which means a child, stepchild, parent, stepparent, spouse, sibling, mother-in-law, father-in-law, son-in-law, daughter-in-law, brother-in-law, sister-in-law or any person sharing the household (other than a tenant or employee);
•any nominee for director and the immediate family members of such nominee; and
•a 5% beneficial owner of the Company’s voting securities or any immediate family member of such owner.
We have policies and procedures designed to minimize potential conflicts of interest arising from any dealings it may have with its affiliates and to provide appropriate procedures for the disclosure of any real or potential conflicts of interest that may exist from time to time. Specifically, pursuant to its audit committee charter, the audit committee has the responsibility to review related party transactions.
Independent Board of Directors
NYSE rules generally require that independent directors must comprise a majority of a listed company’s board of directors. Our Board has determined that Kenneth C. Bockhorst, Robert A. Cascella, Steven W. Etzel, John W. Kuo and Jody A. Markopoulos, representing five (5) of our nine (9) directors, are “independent” as that term is defined under the applicable rules and regulations of the SEC and the listing requirements and rules of the NYSE. Our independent directors hold regularly scheduled meetings at which only independent directors are present.
ITEM 14. PRINCIPAL ACCOUNTING FEES AND SERVICES
The following is a summary of fees paid or to be paid to Deloitte & Touche, LLP ("Deloitte"), for services rendered.
Audit Fees. Audit fees consist of fees billed for professional services rendered for the audit of our year-end financial statements and services that are normally provided by Deloitte in connection with regulatory filings. The aggregate fees billed by Deloitte for professional services rendered for the audit of our annual financial statements, and other required filings with the SEC for the year ended December 31, 2021 and 2020 totaled $6,486,950 and $2,175,950, respectively. The above amounts include interim procedures and audit fees, as well as attendance at audit committee meetings.
Audit-Related Fees. Audit-related services consist of fees billed for assurance and related services that are reasonably related to performance of the audit or review of our financial statements and are not reported under “Audit Fees.” These services include attest services that are not required by statute or regulation and consultations concerning financial accounting and reporting standards. The aggregate fees billed by Deloitte for audit-related professional fees for the year ended December 31, 2021 were $375,000. We did not pay Deloitte audit related fees for the year ended December 31, 2020.
Tax Fees. Deloitte fees for tax planning and tax advisory services for the year ended December 31, 2021 were $2,551,468. We did not pay Deloitte for tax related services in the year ended December 31, 2020.
All Other Fees. We did not pay Deloitte for other services for the years ended December 31, 2021 or 2020.
Pre-Approval Policy
Our audit committee was formed upon the consummation of our Business Combination. As a result, the audit committee did not pre-approve all of the foregoing services, although any services rendered prior to the formation of our audit committee were approved by our board of directors. Since the formation of our audit committee, and on a going-forward basis, the audit committee has and will pre-approve all auditing services and permitted non-audit services to be performed for us by our auditors, including the fees and terms thereof (subject to the de minimis exceptions for non-audit services described in the Exchange Act which are approved by the audit committee prior to the completion of the audit).
PART IV
ITEM 15. EXHIBITS AND FINANCIAL STATEMENT SCHEDULES
1. Financial Statements
See Index to Consolidated Financial Statements appearing in Item 8 “Financial Statements and Supplementary Data” of this Annual Report.
2. Financial Statement Schedules
The following financial statement schedules are included in this Form 10-K:
•Schedule I - Condensed Financial Information of Registrant is presented for the Successor Period from October 20, 2021 through December 31, 2021, the Predecessor Stub Period from July 1, 2021 through October 19, 2021, and Predecessor fiscal years ended June 30, 2021, 2020 and 2019.
•Schedule II - Valuation and Qualifying Accounts is presented for the Successor Period from October 20, 2021 through December 31, 2021, the Predecessor Stub Period from July 1, 2021 through October 19, 2021, and Predecessor fiscal years ended June 30, 2021, 2020 and 2019.
All remaining schedules are omitted and are either inapplicable or not required, or the required information is presented in the financial statements or notes thereto.
3. Exhibits
The exhibits listed on the accompanying Exhibit Index are filed or incorporated by reference as part of this Annual Report.
EXHIBIT INDEX
| | | | | |
Exhibit Number | Exhibit Title |
2.1 | Business Combination Agreement, dated as of June 17, 2021, by and among GS Acquisition Holdings Corp II, Mirion Technologies (TopCo), Ltd., CCP IX LP No. 1, CCP IX LP No. 2, CCP IX Co-Investment LP and CCP IX Co-Investment No. 2 LP, each acting by their general partner, Charterhouse General Partners (IX) Limited and the other parties thereto (incorporated by reference to Exhibit 2.1 to the Company’s Current Report on Form 8-K filed with the SEC on June 21, 2021). |
2.2 | Amendment No. 1 to Business Combination Agreement, dated as of September 2, 2021, by and among GS Acquisition Holdings Corp II, Mirion Technologies (TopCo), Ltd. and CCP IX LP No. 1, CCP IX LP No. 2, CCP IX Co-Investment LP and CCP IX Co-Investment No. 2 LP, each acting by their general partner, Charterhouse General Partners (IX) Limited, on behalf of the Sellers (incorporated by reference to Exhibit 2.1 to the Company’s Current Report on Form 8-K filed with the SEC on September 3, 2021). |
| 3.1 | |
| 3.2 | |
| 4.1 | |
| 4.2 | |
| 4.3 | |
| 4.4* | |
| 10.1 | Credit Agreement, dated as of October 20, 2021, by and between Mirion Technologies (HoldingSub2), Ltd., a limited liability company incorporated in England and Wales, as Holdings, Mirion Technologies (US Holdings), Inc., as the Parent Borrower, Mirion Technologies (US), Inc., as the Subsidiary Borrower, the lending institutions party thereto and Citibank, N.A., as Administrative Agent and Collateral Agent (incorporated by reference to Exhibit 10.1 to the Company’s Current Report on Form 8-K filed with the SEC on October 25, 2021). |
| 10.2* | Amendment to Credit Agreement dated as of November 22, 2021, by and between Mirion Technologies (HoldingSub2), Ltd., a limited liability company incorporated in England and Wales, as Holdings, Mirion Technologies (US Holdings), Inc., as the Parent Borrower, Mirion Technologies (US), Inc., as the Subsidiary Borrower, the lending institutions party thereto and Citibank, N.A., as Administrative Agent and Collateral Agent. |
| 10.3 | |
| 10.4 | Amended and Restated Registration Rights Agreement, dated October 20, 2021, by and among Mirion Technologies, Inc., GS Sponsor II LLC, GS Acquisition Holdings II Employee Participation LLC, GS Acquisition Holdings II Employee Participation 2 LLC, GS II PIPE Investors Employee LP, NRD PIPE Investors LP, the Charterhouse Parties and the Sellers (incorporated by reference to Exhibit 10.3 to the Company’s Current Report on Form 8-K filed with the SEC on October 25, 2021). |
| 10.5 | |
| 10.6 | |
| 10.7 | |
| 10.8* | |
| 10.9* | |
| | | | | |
Exhibit Number | Exhibit Title |
| 10.10* | |
| 10.11 | |
| 10.12 | |
| 10.13 | |
| 10.14 | |
| 10.15 | |
| 10.16 | |
| 10.17 | |
| 10.18 | |
| 10.19 | |
| 10.20 | |
| 10.21 | |
| 10.22 | |
| 10.23 | |
| 10.24 | |
| 10.25 | |
| 10.26 | |
| 10.27 | |
| 10.28 | |
| 10.29 | |
| 10.30 | |
| | | | | |
Exhibit Number | Exhibit Title |
| 14.1 | |
| 16.1 | |
| 21.1* | |
| 23.1* | |
| 24.1* | |
| 31.1* | |
| 31.2* | |
| 32.1* | |
| 32.2* | |
| 101.INS* | XBRL Instance Document. |
| 101.SCH* | XBRL Taxonomy Extension Schema Document. |
| 101.CAL* | XBRL Taxonomy Extension Calculation Linkbase Document. |
| 101.DEF* | XBRL Taxonomy Extension Definition Linkbase Document. |
| 101.LAB* | XBRL Taxonomy Extension Label Linkbase Document. |
| 101.PRE* | XBRL Taxonomy Extension Presentation Linkbase Document. |
| 104 | Cover Page Interactive Data File (formatted as Inline XBRL and contained in Exhibit 101). |
* Filed herewith.
Schedule I - Condensed Financial Information of the Registrant
Successor Period
Mirion Technologies, Inc. has no material assets or standalone operations other than its ownership in its consolidated subsidiaries. There are restrictions under credit agreements governing the 2021 Credit Agreement, described in Note 8, Borrowings, on the Company’s ability to obtain funds from any of its subsidiaries through dividends. Accordingly, the following condensed financial information is presented on a “Parent-only” basis in which Mirion Technologies, Inc.’s investments in its consolidated subsidiaries are presented under the equity method of accounting.
MIRION TECHNOLOGIES, INC.
(PARENT COMPANY ONLY)
CONDENSED BALANCE SHEET
(in millions)
| | | | | |
| Successor |
| December 31,
2021 |
| Assets: | |
| Investments in Sub | $ | 1,851.1 | |
| Total Assets | $ | 1,851.1 | |
| |
| Liabilities and Stockholders’ Equity: | |
| Warrant liabilities | 68.1 | |
| Deferred income taxes and other liabilities | (1.0) | |
| Total Liabilities | 67.1 | |
| Additional paid-in capital | 1,845.5 | |
| Accumulated deficit | (131.6) | |
| Accumulated Other Comprehensive Loss | (20.7) | |
| Mirion Technologies, Inc. (Successor) stockholders’ equity | 1,693.2 | |
| Noncontrolling interests | 90.8 | |
| Total Stockholders’ Equity | 1,784.0 | |
| Total Liabilities and Stockholders’ Equity | $ | 1,851.1 | |
MIRION TECHNOLOGIES, INC.
(PARENT COMPANY ONLY)
CONDENSED STATEMENT OF OPERATIONS AND COMPREHENSIVE LOSS INCOME
(in millions)
| | | | | |
| Successor |
| From
October 20, 2021 through
December 31, 2021 |
| Selling, general and administrative | $ | 5.3 | |
| Total operating expenses | 5.3 | |
| Income from operations | (5.3) | |
| Change in fair value of warrant liabilities | (1.2) | |
| Equity in net loss of subsidiaries | 19.9 | |
| Loss before benefit from income taxes | $ | (24.0) | |
| Benefit from income taxes | (1.0) | |
| Net loss | $ | (23.0) | |
| Loss attributable to noncontrolling interests | (0.8) | |
| Net loss attributable to Mirion Technologies, Inc. stockholders | (22.2) | |
| Foreign currency translation, net of tax | (20.5) | |
| Unrecognized actuarial gain (loss) and prior service benefit, net of tax | (0.2) | |
| Other comprehensive loss (income), net of tax | (20.7) | |
Comprehensive loss attributable to Mirion Technologies, Inc. stockholders | (42.9) | |
| Loss per share—basic and diluted | (0.12) | |
| Weighted average number of shares outstanding—basic and diluted | 180.773 | |
A statement of cash flows has not been presented as Mirion Technologies, Inc. parent company did not have any cash as of, or at any point in time during the year ended December 31, 2021, other than cash and cash equivalents held in escrow on October 19, 2021 ($750.2 million) that was used to fund the Mirion Business Combination and to fund the redemption of redeemable Class A shares of Common Stock ($146.3 million) on October 20, 2021.
Note to Condensed Financial Statements of Registrant (Parent Company Only)
Basis of Presentation
These condensed parent company-only financial statements have been prepared in accordance with Rule 12-04, Schedule I of Regulation S-X, as the restricted net assets of the subsidiaries of Mirion. (as defined in Rule 4-08(e)(3) of Regulation S-X) exceed the specified threshold amount of the consolidated net assets of the Company. Because we have a consolidated accumulated deficit, the 25% threshold described in Rule 4-08 does not apply and any restrictions of net assets at our subsidiaries trigger the requirement to present parent company-only financial information. The ability of Mirion’s operating subsidiaries to pay dividends may be restricted due to the terms of the subsidiaries’ outstanding term loan and revolving credit facility borrowings as described in Note 8 to the audited consolidated financial statements.
These condensed parent company-only financial statements have been prepared using the same accounting principles and policies described in the notes to the consolidated financial statements, with the only exception being that the parent company accounts for its subsidiaries using the equity method. These condensed parent company-only financial statements should be read in conjunction with the consolidated financial statements and related notes.
Predecessor Period:
Mirion Technologies (TopCo), Ltd. has no material assets or standalone operations other than its ownership in its consolidated subsidiaries. There are restrictions under credit agreements governing the 2019 Credit Facility, described in Note 8, on the Company’s ability to obtain funds from any of its subsidiaries through dividends. Accordingly, the following condensed financial information is presented on a “Parent-only” basis in which Mirion Technologies (TopCo), Ltd.’s investments in its consolidated subsidiaries are presented under the equity method of accounting.
MIRION TECHNOLOGIES, INC.
(PARENT COMPANY ONLY)
CONDENSED BALANCE SHEET
(in millions)
| | | | | | | | | | | |
| June 30,
2021 | | June 30,
2020 |
| Assets: | | | |
| Other assets | $ | 0.3 | | | $ | 0.1 | |
| Total assets | $ | 0.3 | | | $ | 0.1 | |
| | | |
| Liabilities and stockholders’ equity: | | | |
| Loan from subsidiary | $ | 839.8 | | | $ | 716.5 | |
| Deferred income taxes and other liabilities | 0.1 | | | 0.1 | |
| Total Liabilities | $ | 839.9 | | | $ | 716.6 | |
| Total stockholders’ equity | (839.6) | | | (716.5) | |
| Total liabilities and stockholders’ equity | $ | 0.3 | | | $ | 0.1 | |
MIRION TECHNOLOGIES, INC.
(PARENT COMPANY ONLY)
CONDENSED STATEMENT OF OPERATIONS AND COMPREHENSIVE LOSS INCOME
(in millions)
| | | | | | | | | | | | | | | | | | | | | | | |
| From July 1, 2021 through
October 19, 2021 | | June 30,
2021 | | June 30,
2020 | | June 30,
2019 |
| Equity in net loss of subsidiaries | $ | (105.7) | | | $ | (158.3) | | | $ | (119.1) | | | $ | (122.0) | |
| Net loss | (105.7) | | | (158.3) | | | (119.1) | | | (122.0) | |
| Foreign currency translation, net of tax | (7.5) | | | 34.2 | | | (9.3) | | | (15.1) | |
| Unrecognized actuarial gain (loss) and prior service benefit, net of tax | 0.6 | | | 0.9 | | | — | | | (1.5) | |
| Other comprehensive loss (income), net of tax | (6.9) | | | 35.1 | | | (9.3) | | | (16.6) | |
| Comprehensive loss | $ | (112.6) | | | $ | (123.2) | | | $ | (128.4) | | | $ | (138.6) | |
| Loss per share—basic and diluted | $ | (15.81) | | | $ | (24.18) | | | $ | (18.45) | | | $ | (19.36) | |
| Weighted average number of shares outstanding—basic and diluted | 6.685 | | | 6.549 | | | 6.453 | | | 6.300 | |
A statement of cash flows has not been presented as Mirion Technologies (TopCo). Ltd. parent company did not have any cash as of, or at any point in time during the Predecessor Periods from July 1, 2021 through October 19, 2021 or the years ended June 30, 2021, 2020 or 2019.
Note to Condensed Financial Statements of Registrant (Parent Company Only)
Basis of Presentation
These condensed parent company-only financial statements have been prepared in accordance with Rule 12-04, Schedule I of Regulation S-X, as the restricted net assets of the subsidiaries of Mirion. (as defined in Rule 4-08(e)(3) of Regulation S-X) exceed the specified threshold amount of the consolidated net assets of the Company. Because we have a consolidated
accumulated deficit, the 25% threshold described in Rule 4-08 does not apply and any restrictions of net assets at our subsidiaries trigger the requirement to present parent company-only financial information. The ability of Mirion’s operating subsidiaries to pay dividends may be restricted due to the terms of the subsidiaries’ outstanding term loan and revolving credit facility borrowings as described in Note 8, Borrowings, to the audited consolidated financial statements.
These condensed parent company-only financial statements have been prepared using the same accounting principles and policies described in the notes to the consolidated financial statements, with the only exception being that the parent company accounts for its subsidiaries using the equity method. These condensed parent company-only financial statements should be read in conjunction with the consolidated financial statements and related notes.
Schedule II
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Valuation and Qualifying Accounts |
| (In millions) |
| Successor |
| | Balance at | | Charged to | | | | | | Balance at |
| | Beginning | | Costs and | | Deductions | | | | End of |
| Description | | of Period | | Expenses | | (a) | | Other (b) | | Period |
| Year Ended December 31, 2021 | | | | | | | | | | |
| Allowance for doubtful accounts | | $ | — | | | $ | — | | | $ | (0.7) | | | $ | 6.1 | | | $ | 5.4 | |
| Product warranty | | — | | | 0.9 | | | (0.4) | | | 5.4 | | | 5.9 | |
| | | | | | | | | | |
| Predecessor |
| | Balance at | | Charged to | | | | | | Balance at |
| | Beginning | | Costs and | | Deductions | | | | End of |
| Description | | of Period | | Expenses | | (a) | | Other (b) | | Period |
| Period Ended October 19, 2021 | | | | | | | | | | |
| Allowance for doubtful accounts | | $ | 6.1 | | | $ | 0.2 | | | $ | (0.2) | | | $ | — | | | $ | 6.1 | |
| Product warranty | | 6.3 | | | — | | | (0.7) | | | — | | | 5.6 | |
| Year Ended June 30, 2021 | | | | | | | | | | |
| Allowance for doubtful accounts | | $ | 1.9 | | | $ | 2.5 | | | $ | (0.7) | | | $ | 2.4 | | | $ | 6.1 | |
| Product warranty | | 5.5 | | | 2.8 | | | (2.2) | | | 0.2 | | | 6.3 | |
| Year Ended June 30, 2020 | | | | | | | | | | |
| Allowance for doubtful accounts | | $ | 1.7 | | | $ | 0.9 | | | $ | (0.7) | | | $ | — | | | $ | 1.9 | |
| Product warranty | | 4.2 | | | 2.9 | | | (1.6) | | | — | | | 5.5 | |
| Year Ended June 30, 2019 | | | | | | | | | | |
| Allowance for doubtful accounts | | $ | 1.9 | | | $ | 0.2 | | | $ | (0.4) | | | $ | — | | | $ | 1.7 | |
| Product warranty | | 5.4 | | | 1.5 | | | (2.6) | | | (0.1) | | | 4.2 | |
(a)Charges to the accounts included in this column are for the purposes for which the reserves were created
(b)Amounts included in this column relate to foreign currency translation and valuation adjustments from business combinations
ITEM 16. FORM 10-K SUMMARY
None.
SIGNATURES AND POWER OF ATTORNEY
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto duly authorized.
Date: February 28, 2022
| | | | | | | | |
| MIRION TECHNOLOGIES, INC. |
| |
| By | | /s/ Thomas D. Logan |
| | Name: Thomas D. Logan |
| | Title: Chief Executive Officer and Director |
KNOW ALL PERSONS BY THESE PRESENTS, that each person whose signature appears below constitutes and appoints Thomas D. Logan, Brian Schopfer and Emmanuelle Lee and each of them, his or her true and lawful attorneys-in-fact and agents, with full power of substitution and resubstitution, for him or her and in his or her name, place and stead, in any and all capacities, to sign any and all amendments to this Annual Report on Form 10-K, and to file the same, with all exhibits thereto, and all other documents in connection therewith, with the Securities and Exchange Commission, granting unto each said attorney-in-fact and agents full power and authority to do and perform each and every act in person, hereby ratifying and confirming all that said attorneys-in-fact and agents or either of them or their or his or her substitute or substitutes may lawfully do or cause to be done by virtue hereof.
Pursuant to the requirements of the Securities Exchange Act of 1934, this report has been signed by the following persons on behalf of the registrant and in the capacities and on the dates indicated.
| | | | | | | | |
| Name | Title | Date |
| | |
/s/ Thomas D. Logan Thomas D. Logan | Chief Executive Officer and Director (principal executive officer) | February 28, 2022 |
| | |
/s/ Brian Schopfer Brian Schopfer | Chief Financial Officer (principal financial officer) | February 28, 2022 |
| | |
/s/ Kipling Matas Kipling Matas | Chief Accounting Officer (principal accounting officer) | February 28, 2022 |
| | |
/s/ Lawrence D. Kingsley Lawrence D. Kingsley | Director and Chairman | February 28, 2022 |
| | |
/s/ Jyothsna Natauri Jyothsna Natauri | Director | February 28, 2022 |
| | |
/s/ Christopher Warren Christopher Warren | Director | February 28, 2022 |
| | |
/s/ Steven W. Etzel Steven W. Etzel | Director | February 28, 2022 |
| | |
/s/ Kenneth C. Bockhorst Kenneth C. Bockhorst | Director | February 28, 2022 |
| | | | | | | | |
| Name | Title | Date |
| | |
/s/ Robert A. Cascella Robert A. Cascella | Director | February 28, 2022 |
| | |
/s/ John W. Kuo John W. Kuo | Director | February 28, 2022 |
| | |
/s/ Jody A. Markopoulos Jody A. Markopoulos | Director | February 28, 2022 |