EX-99.2
Published on June 17, 2021
June 2021 Investor Presentation Exhibit 99.2

Disclaimer This investor presentation (the “presentation”) is for informational purposes only and does not constitute an offer to sell, a solicitation of an offer to buy, or a recommendation to purchase any equity, debt or other financial instruments of GS Acquisition Holdings Corp II (“GSAH”) or Mirion Technologies (TopCo), Ltd. (“Mirion”) or any of their respective affiliates. The presentation has been prepared to assist parties in making their own evaluation with respect to a potential business combination between GSAH and Mirion (the “Business Combination”), and for no other purpose. This presentation and the related oral commentary is confidential and is to be maintained in strict confidence. In addition, this presentation is intended solely for investors that are, and by proceeding to participate in this presentation you confirm that you are, qualified institutional buyers or institutions that are accredited investors (as such terms are defined under the rules of the U.S. Securities and Exchange Commission (the “SEC”)). No Offer or Solicitation This presentation shall not constitute a solicitation of a proxy, consent or authorization with respect to any securities or in respect of the Business Combination. This presentation shall also not constitute an offer to sell or the solicitation of an offer to buy any securities pursuant to the Business Combination or otherwise, nor shall there be any sale of securities in any jurisdiction in which the offer, solicitation or sale would be unlawful prior to the registration or qualification under the securities laws of any such jurisdiction. Forward-looking statements This presentation contains “forward-looking statements” within the meaning of the U.S. Private Securities Litigation Reform Act of 1995. Forward-looking statements include, without limitation, statements regarding the estimated future financial performance, financial position and financial impacts of the Business Combination, the timing of the completion of the Business Combination, the implied pro forma enterprise value of the combined company following the Business Combination, anticipated ownership percentages of the combined company’s equityholders following the potential transaction, industry developments and the business strategy, plans and objectives of management for future operations, including as they relate to potential mergers and acquisitions and the potential Business Combination. Such statements can be identified by the fact that they do not relate strictly to historical or current facts. When used in this presentation, words such as “pro forma,” “anticipate,” “believe,” “continue,” “could,” “estimate,” “expect,” “intend,” “may,” “might,” “plan,” “possible,” “potential,” “predict,” “project,” “should,” “strive,” “would” and similar expressions may identify forward-looking statements, but the absence of these words does not mean that a statement is not forward-looking. When GSAH or Mirion discuss their strategies or plans, including as they relate to the Business Combination, they are making projections and forward-looking statements. Such statements are based on the beliefs of, as well as assumptions made by and information currently available to, GSAH’s and Mirion’s management. These forward-looking statements involve significant risk and uncertainties that could cause the actual results to differ materially from the expected results. Most of these factors are outside GSAH’s and Mirion’s control and are difficult to predict. Factors that may cause such differences include, but are not limited to: (1) GSAH’s ability to complete the Business Combination or, if GSAH does not complete the Business Combination, any other initial business combination; (2) satisfaction or waiver (if applicable) of the conditions to the Business Combination, including with respect to the approval of the stockholders of GSAH; (3) the ability to maintain the listing of the combined company’s securities on the New York Stock Exchange; (4) the inability to complete the PIPE Investment; (5) the risk that the Business Combination disrupts current plans and operations of GSAH or Mirion as a result of the announcement and consummation of the transaction described herein; (6) the ability to recognize the anticipated benefits of the Business Combination, which may be affected by, among other things, competition, the ability of the combined company to grow and manage growth profitably, maintain relationships with customers and suppliers and retain its management and key employees; (7) costs related to the Business Combination; (8) changes in applicable laws or regulations and delays in obtaining, adverse conditions contained in, or the inability to obtain necessary regulatory approvals required to complete the Business Combination; (9) the possibility that Mirion and GSAH may be adversely affected by other economic, business, and/or competitive factors; (10) the outcome of any legal proceedings that may be instituted against GSAH, Mirion or any of their respective directors or officers following the announcement of the Business Combination; (11) the failure to realize anticipated pro forma and projected results and underlying assumptions, including with respect to estimated stockholder redemptions and purchase price and other adjustments; (12) future global, regional or local political, market and social conditions, including due to the COVID-19 pandemic; and (13) other risks and uncertainties included in the Risk Factors at the end of this presentation, GSAH’s final prospectus relating to its initial public offering dated June 29, 2020 filed with the SEC under the heading "Risk Factors," and other documents GSAH filed, or will file, with the SEC, available at www.sec.gov. You are cautioned not to place undue reliance upon any forward-looking statements. Forward-looking statements included in this presentation speak only as of the date of this presentation. Neither GSAH nor Mirion undertakes any obligation to update its forward-looking statements to reflect events or circumstances after the date hereof. No Representation or Warranty No representation or warranty, express or implied, is or will be given by GSAH, Mirion, any of their respective subsidiaries, equityholders, affiliates or any of the representatives, partners, directors, officers, employees, advisers or agents of GSAH, Mirion or any of their respective subsidiaries, equityholders or affiliates, or any other person, as to the accuracy or completeness of the information in this presentation or any other written, oral or other communications transmitted or otherwise made available to any party in the course of its evaluation of the Business Combination, and no responsibility or liability whatsoever is accepted by any such person for the accuracy or sufficiency thereof or for any errors, omissions or misstatements, negligent or otherwise, relating thereto. This presentation does not purport to contain all of the information that may be required to evaluate a possible investment decision with respect to GSAH, and does not constitute investment, tax or legal advice. The recipient also acknowledges and agrees that the information contained in this presentation is preliminary in nature and is subject to change, and any such changes may be material. GSAH and Mirion disclaim any duty to update the information contained in this presentation. Any and all trademarks and trade names referred to in this presentation are the property of their respective owners.

Disclaimer Industry and Market Data In this presentation, we rely on and refer to information and statistics regarding market participants in the sectors in which Mirion competes and other industry data. We obtained this information and statistics from third-party sources, including reports by market research firms and company filings. Neither GSAH nor Mirion has independently verified the data obtained from these sources and cannot assure you of the data’s accuracy or completeness. Use of Projections This presentation contains projected financial information. Neither GSAH’s nor Mirion’s independent auditors have studied, reviewed, compiled or performed any procedures with respect to the projections for the purpose of their inclusion in this presentation, and accordingly, neither of them expressed an opinion or provided any other form of assurance with respect thereto for the purpose of this presentation. These projections are for illustrative purposes only and should not be relied upon as being necessarily indicative of future results. In this presentation, certain of the above-mentioned projected information has been provided for purposes of providing comparisons with historical data. The assumptions and estimates underlying the prospective financial information are inherently uncertain and are subject to a wide variety of significant business, economic and competitive risks and uncertainties that could cause actual results to differ materially from those contained in the prospective financial information. Projections are inherently uncertain due to a number of factors outside of GSAH’s or Mirion’s control. Accordingly, there can be no assurance that the prospective results are indicative of future performance of GSAH, Mirion or the combined company after the Business Combination or that actual results will not differ materially from those presented in the prospective financial information. Inclusion of the prospective financial information in this presentation should not be regarded as a representation by any person that the results contained in the prospective financial information will be achieved. Non-GAAP Financial Matters This presentation includes certain non-GAAP financial measures, including Adjusted Revenue, Adjusted EBITDA, Adjusted EBITDA Less Maintenance CapEx, Return on Invested Capital and Free Cash Flow Conversion, that are not prepared in accordance with accounting principles generally accepted in the United States (“GAAP”) and that may be different from non-GAAP financial measures used by other companies and not directly comparable. GSAH and Mirion believe that the use of these non-GAAP financial measures provides an additional tool for investors to use in evaluating ongoing operating results and trends. These non-GAAP measures with comparable names should not be considered in isolation from, or as an alternative to, financial measures determined in accordance with GAAP. See the footnotes on the slides where these measures are discussed and the Non-GAAP reconciliations beginning on slide 39 for a description of these non-GAAP financial measures and reconciliations of such non-GAAP financial measures to the most comparable GAAP amounts. Additionally, to the extent that forward-looking non-GAAP financial measures are provided, they are presented on a non-GAAP basis without reconciliations of such forward-looking non-GAAP measures due to the inherent difficulty in projecting and quantifying the various adjusting items necessary for such reconciliations that have not yet occurred, are out of GSAH’s and Mirion’s control or cannot be reasonably predicted. Trademarks This presentation contains trademarks, service marks, trade names and copyrights of GSAH, Mirion and other companies, which are the property of their respective owners.
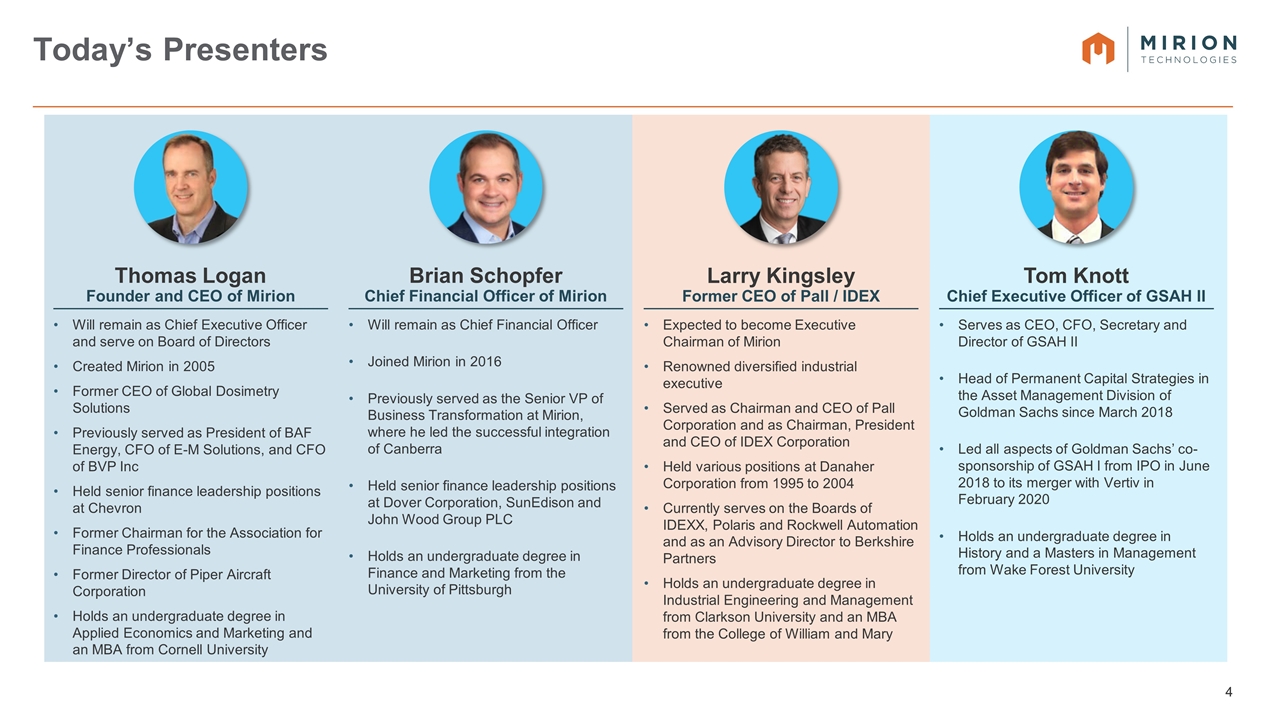
Today’s Presenters Larry Kingsley Former CEO of Pall / IDEX Expected to become Executive Chairman of Mirion Renowned diversified industrial executive Served as Chairman and CEO of Pall Corporation and as Chairman, President and CEO of IDEX Corporation Held various positions at Danaher Corporation from 1995 to 2004 Currently serves on the Boards of IDEXX, Polaris and Rockwell Automation and as an Advisory Director to Berkshire Partners Holds an undergraduate degree in Industrial Engineering and Management from Clarkson University and an MBA from the College of William and Mary Tom Knott Chief Executive Officer of GSAH II Serves as CEO, CFO, Secretary and Director of GSAH II Head of Permanent Capital Strategies in the Asset Management Division of Goldman Sachs since March 2018 Led all aspects of Goldman Sachs’ co-sponsorship of GSAH I from IPO in June 2018 to its merger with Vertiv in February 2020 Holds an undergraduate degree in History and a Masters in Management from Wake Forest University Brian Schopfer Chief Financial Officer of Mirion Will remain as Chief Financial Officer Joined Mirion in 2016 Previously served as the Senior VP of Business Transformation at Mirion, where he led the successful integration of Canberra Held senior finance leadership positions at Dover Corporation, SunEdison and John Wood Group PLC Holds an undergraduate degree in Finance and Marketing from the University of Pittsburgh Thomas Logan Founder and CEO of Mirion Will remain as Chief Executive Officer and serve on Board of Directors Created Mirion in 2005 Former CEO of Global Dosimetry Solutions Previously served as President of BAF Energy, CFO of E-M Solutions, and CFO of BVP Inc Held senior finance leadership positions at Chevron Former Chairman for the Association for Finance Professionals Former Director of Piper Aircraft Corporation Holds an undergraduate degree in Applied Economics and Marketing and an MBA from Cornell University
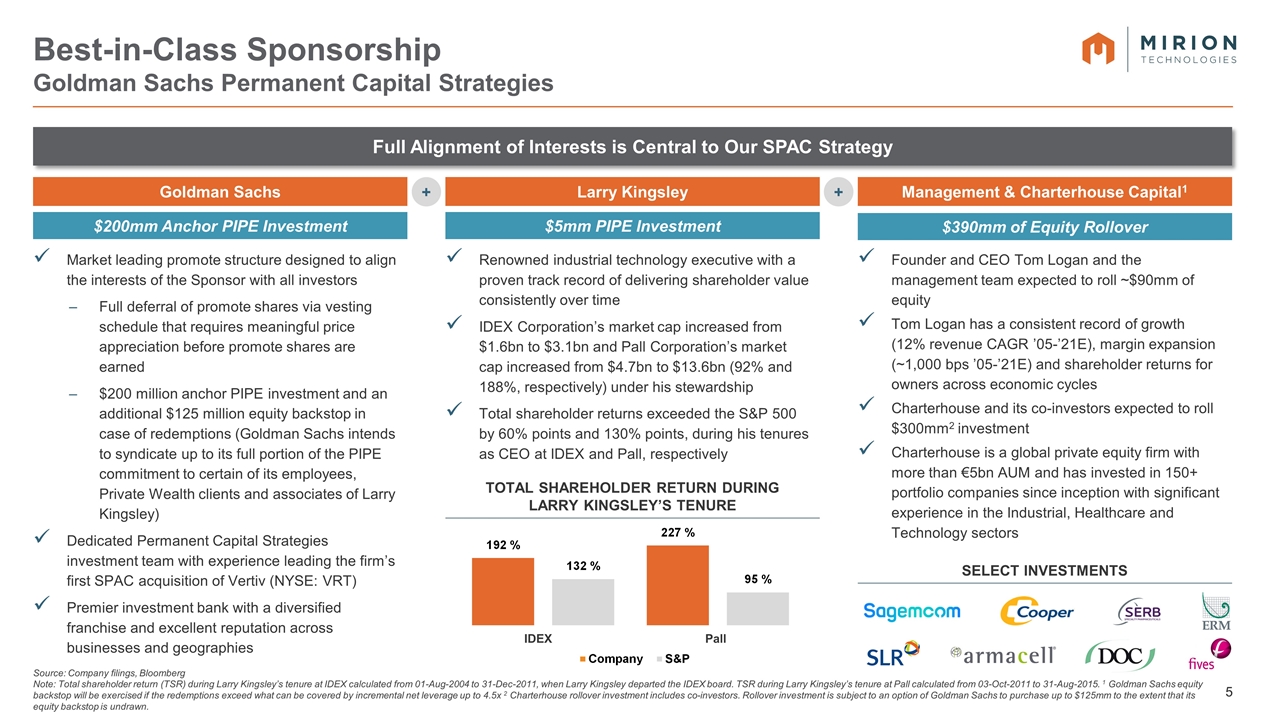
Management & Charterhouse Capital1 $5mm PIPE Investment Larry Kingsley Best-in-Class Sponsorship Goldman Sachs Permanent Capital Strategies Source: Company filings, Bloomberg Note: Total shareholder return (TSR) during Larry Kingsley’s tenure at IDEX calculated from 01-Aug-2004 to 31-Dec-2011, when Larry Kingsley departed the IDEX board. TSR during Larry Kingsley’s tenure at Pall calculated from 03-Oct-2011 to 31-Aug-2015. 1 Goldman Sachs equity backstop will be exercised if the redemptions exceed what can be covered by incremental net leverage up to 4.5x 2 Charterhouse rollover investment includes co-investors. Rollover investment is subject to an option of Goldman Sachs to purchase up to $125mm to the extent that its equity backstop is undrawn. + + Full Alignment of Interests is Central to Our SPAC Strategy $200mm Anchor PIPE Investment $390mm of Equity Rollover Market leading promote structure designed to align the interests of the Sponsor with all investors Full deferral of promote shares via vesting schedule that requires meaningful price appreciation before promote shares are earned $200 million anchor PIPE investment and an additional $125 million equity backstop in case of redemptions (Goldman Sachs intends to syndicate up to its full portion of the PIPE commitment to certain of its employees, Private Wealth clients and associates of Larry Kingsley) Dedicated Permanent Capital Strategies investment team with experience leading the firm’s first SPAC acquisition of Vertiv (NYSE: VRT) Premier investment bank with a diversified franchise and excellent reputation across businesses and geographies Goldman Sachs Renowned industrial technology executive with a proven track record of delivering shareholder value consistently over time IDEX Corporation’s market cap increased from $1.6bn to $3.1bn and Pall Corporation’s market cap increased from $4.7bn to $13.6bn (92% and 188%, respectively) under his stewardship Total shareholder returns exceeded the S&P 500 by 60% points and 130% points, during his tenures as CEO at IDEX and Pall, respectively total shareholder return during Larry Kingsley’s tenure Select Investments IDEX Pall Founder and CEO Tom Logan and the management team expected to roll ~$90mm of equity Tom Logan has a consistent record of growth (12% revenue CAGR ’05-’21E), margin expansion (~1,000 bps ’05-’21E) and shareholder returns for owners across economic cycles Charterhouse and its co-investors expected to roll $300mm2 investment Charterhouse is a global private equity firm with more than €5bn AUM and has invested in 150+ portfolio companies since inception with significant experience in the Industrial, Healthcare and Technology sectors
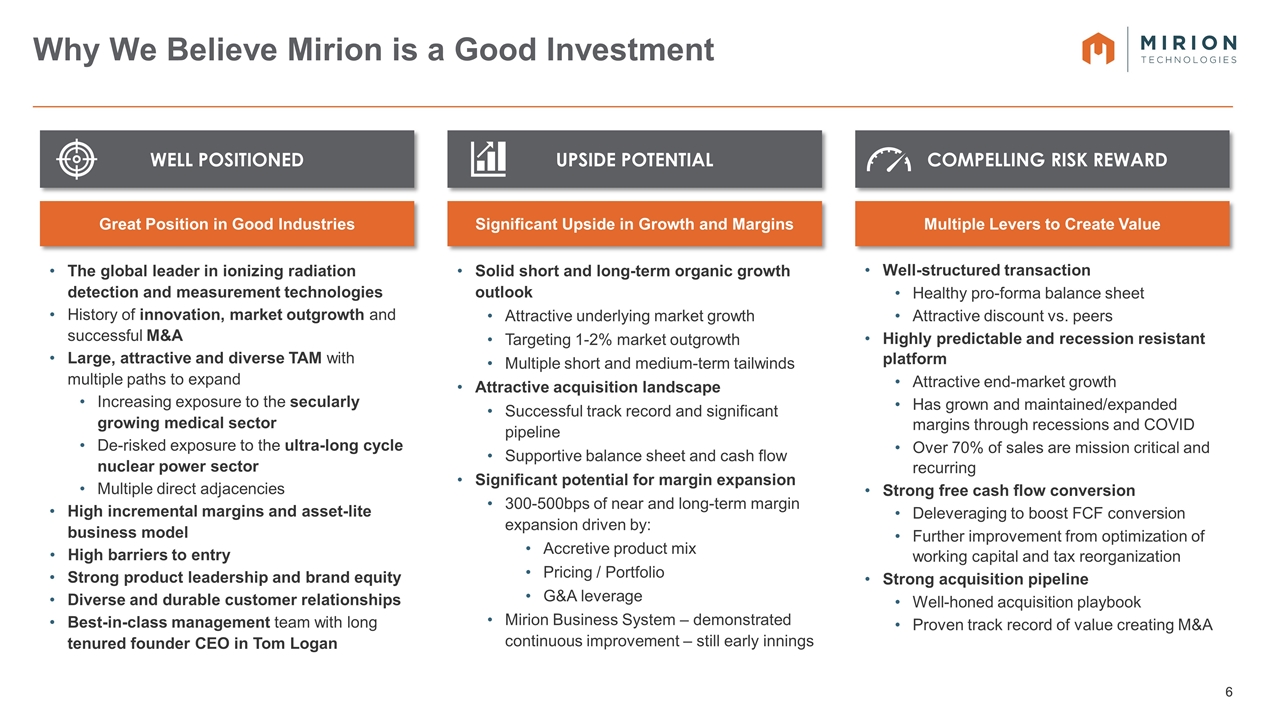
Why We Believe Mirion is a Good Investment The global leader in ionizing radiation detection and measurement technologies History of innovation, market outgrowth and successful M&A Large, attractive and diverse TAM with multiple paths to expand Increasing exposure to the secularly growing medical sector De-risked exposure to the ultra-long cycle nuclear power sector Multiple direct adjacencies High incremental margins and asset-lite business model High barriers to entry Strong product leadership and brand equity Diverse and durable customer relationships Best-in-class management team with long tenured founder CEO in Tom Logan WELL POSITIONED Solid short and long-term organic growth outlook Attractive underlying market growth Targeting 1-2% market outgrowth Multiple short and medium-term tailwinds Attractive acquisition landscape Successful track record and significant pipeline Supportive balance sheet and cash flow Significant potential for margin expansion 300-500bps of near and long-term margin expansion driven by: Accretive product mix Pricing / Portfolio G&A leverage Mirion Business System – demonstrated continuous improvement – still early innings UPSIDE POTENTIAL Well-structured transaction Healthy pro-forma balance sheet Attractive discount vs. peers Highly predictable and recession resistant platform Attractive end-market growth Has grown and maintained/expanded margins through recessions and COVID Over 70% of sales are mission critical and recurring Strong free cash flow conversion Deleveraging to boost FCF conversion Further improvement from optimization of working capital and tax reorganization Strong acquisition pipeline Well-honed acquisition playbook Proven track record of value creating M&A COMPELLING RISK REWARD Great Position in Good Industries Significant Upside in Growth and Margins Multiple Levers to Create Value
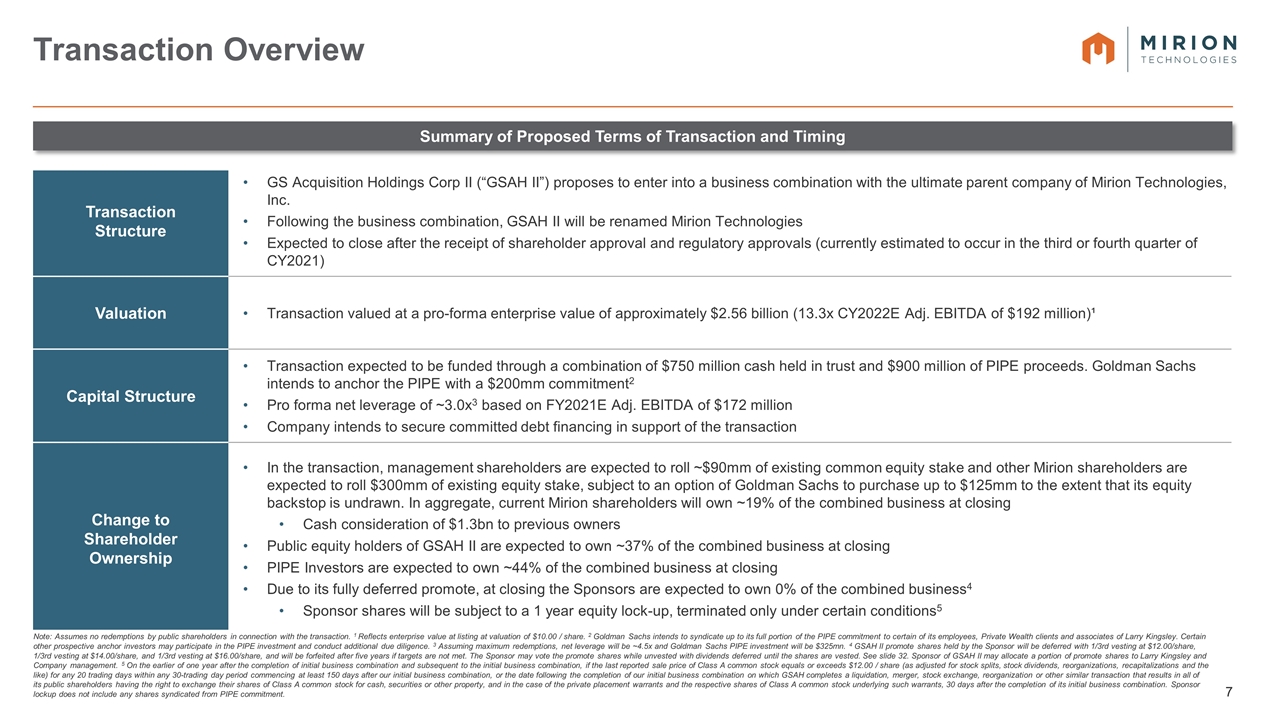
Note: Assumes no redemptions by public shareholders in connection with the transaction. 1 Reflects enterprise value at listing at valuation of $10.00 / share. 2 Goldman Sachs intends to syndicate up to its full portion of the PIPE commitment to certain of its employees, Private Wealth clients and associates of Larry Kingsley. Certain other prospective anchor investors may participate in the PIPE investment and conduct additional due diligence. 3 Assuming maximum redemptions, net leverage will be ~4.5x and Goldman Sachs PIPE investment will be $325mn. 4 GSAH II promote shares held by the Sponsor will be deferred with 1/3rd vesting at $12.00/share, 1/3rd vesting at $14.00/share, and 1/3rd vesting at $16.00/share, and will be forfeited after five years if targets are not met. The Sponsor may vote the promote shares while unvested with dividends deferred until the shares are vested. See slide 32. Sponsor of GSAH II may allocate a portion of promote shares to Larry Kingsley and Company management. 5 On the earlier of one year after the completion of initial business combination and subsequent to the initial business combination, if the last reported sale price of Class A common stock equals or exceeds $12.00 / share (as adjusted for stock splits, stock dividends, reorganizations, recapitalizations and the like) for any 20 trading days within any 30-trading day period commencing at least 150 days after our initial business combination, or the date following the completion of our initial business combination on which GSAH completes a liquidation, merger, stock exchange, reorganization or other similar transaction that results in all of its public shareholders having the right to exchange their shares of Class A common stock for cash, securities or other property, and in the case of the private placement warrants and the respective shares of Class A common stock underlying such warrants, 30 days after the completion of its initial business combination. Sponsor lockup does not include any shares syndicated from PIPE commitment. Transaction Overview Summary of Proposed Terms of Transaction and Timing Transaction Structure GS Acquisition Holdings Corp II (“GSAH II”) proposes to enter into a business combination with the ultimate parent company of Mirion Technologies, Inc. Following the business combination, GSAH II will be renamed Mirion Technologies Expected to close after the receipt of shareholder approval and regulatory approvals (currently estimated to occur in the third or fourth quarter of CY2021) Valuation Transaction valued at a pro-forma enterprise value of approximately $2.56 billion (13.3x CY2022E Adj. EBITDA of $192 million)¹ Capital Structure Transaction expected to be funded through a combination of $750 million cash held in trust and $900 million of PIPE proceeds. Goldman Sachs intends to anchor the PIPE with a $200mm commitment2 Pro forma net leverage of ~3.0x3 based on FY2021E Adj. EBITDA of $172 million Company intends to secure committed debt financing in support of the transaction Change to Shareholder Ownership In the transaction, management shareholders are expected to roll ~$90mm of existing common equity stake and other Mirion shareholders are expected to roll $300mm of existing equity stake, subject to an option of Goldman Sachs to purchase up to $125mm to the extent that its equity backstop is undrawn. In aggregate, current Mirion shareholders will own ~19% of the combined business at closing Cash consideration of $1.3bn to previous owners Public equity holders of GSAH II are expected to own ~37% of the combined business at closing PIPE Investors are expected to own ~44% of the combined business at closing Due to its fully deferred promote, at closing the Sponsors are expected to own 0% of the combined business4 Sponsor shares will be subject to a 1 year equity lock-up, terminated only under certain conditions5
I. Company Overview
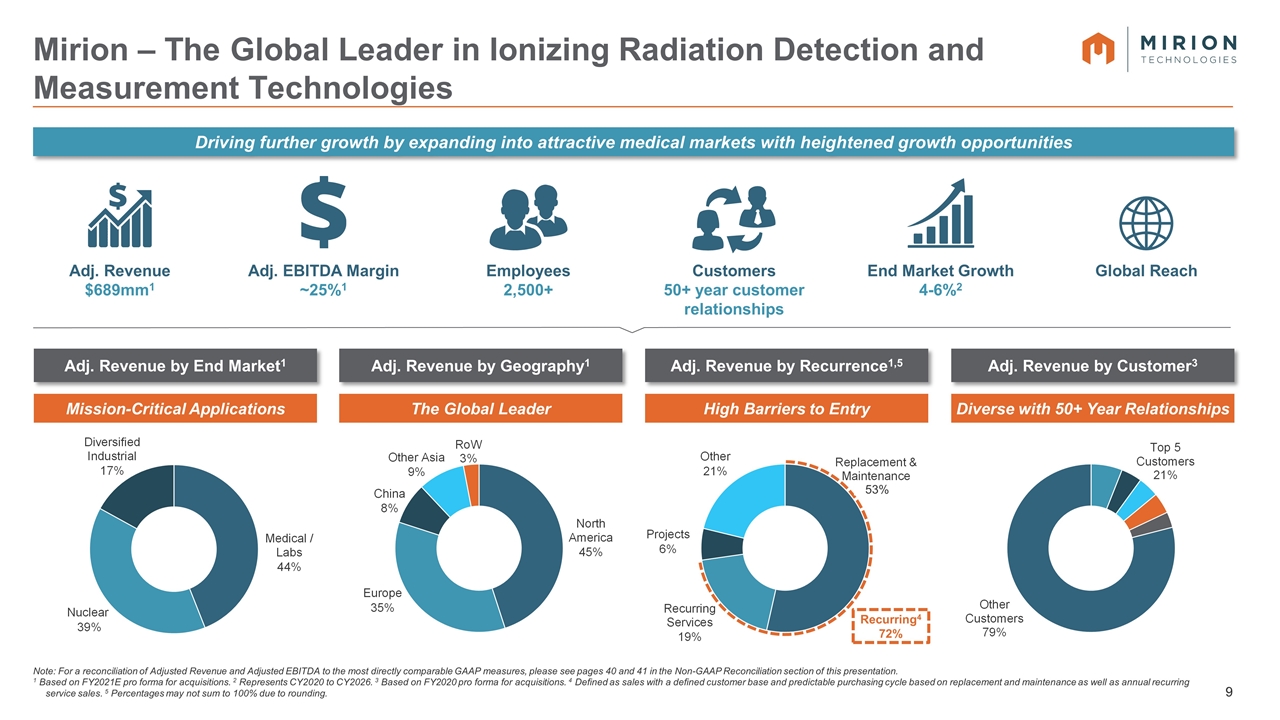
Note: For a reconciliation of Adjusted Revenue and Adjusted EBITDA to the most directly comparable GAAP measures, please see pages 40 and 41 in the Non-GAAP Reconciliation section of this presentation. 1 Based on FY2021E pro forma for acquisitions. 2 Represents CY2020 to CY2026. 3 Based on FY2020 pro forma for acquisitions. 4 Defined as sales with a defined customer base and predictable purchasing cycle based on replacement and maintenance as well as annual recurring service sales. 5 Percentages may not sum to 100% due to rounding. Mirion – The Global Leader in Ionizing Radiation Detection and Measurement Technologies Adj. Revenue $689mm1 Employees 2,500+ Customers 50+ year customer relationships Global Reach Adj. EBITDA Margin ~25%1 End Market Growth 4-6%2 Adj. Revenue by End Market1 Adj. Revenue by Geography1 Adj. Revenue by Recurrence1,5 Adj. Revenue by Customer3 Mission-Critical Applications High Barriers to Entry The Global Leader Diverse with 50+ Year Relationships Recurring4 72% 53% Driving further growth by expanding into attractive medical markets with heightened growth opportunities
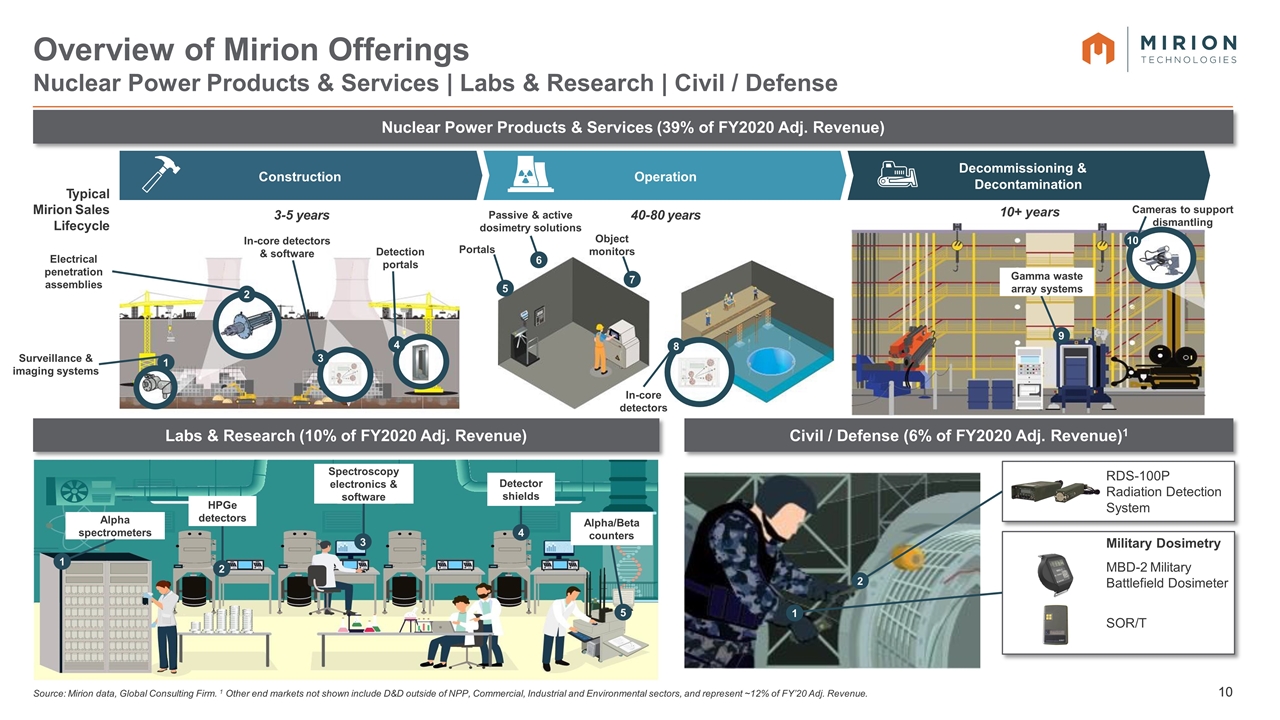
Source: Mirion data, Global Consulting Firm. 1 Other end markets not shown include D&D outside of NPP, Commercial, Industrial and Environmental sectors, and represent ~12% of FY’20 Adj. Revenue. Overview of Mirion Offerings Nuclear Power Products & Services | Labs & Research | Civil / Defense Labs & Research (10% of FY2020 Adj. Revenue) Construction Operation Decommissioning & Decontamination Typical Mirion Sales Lifecycle 1 3 4 5 6 7 8 s 10 9 3-5 years 40-80 years 10+ years Nuclear Power Products & Services (39% of FY2020 Adj. Revenue) Surveillance & imaging systems Electrical penetration assemblies In-core detectors & software Detection portals Portals Passive & active dosimetry solutions Object monitors In-core detectors Gamma waste array systems Cameras to support dismantling Alpha spectrometers Civil / Defense (6% of FY2020 Adj. Revenue)1 Military Dosimetry MBD-2 Military Battlefield Dosimeter SOR/T RDS-100P Radiation Detection System 2 HPGe detectors Spectroscopy electronics & software Detector shields Alpha/Beta counters 1 2 3 4 5 1 2
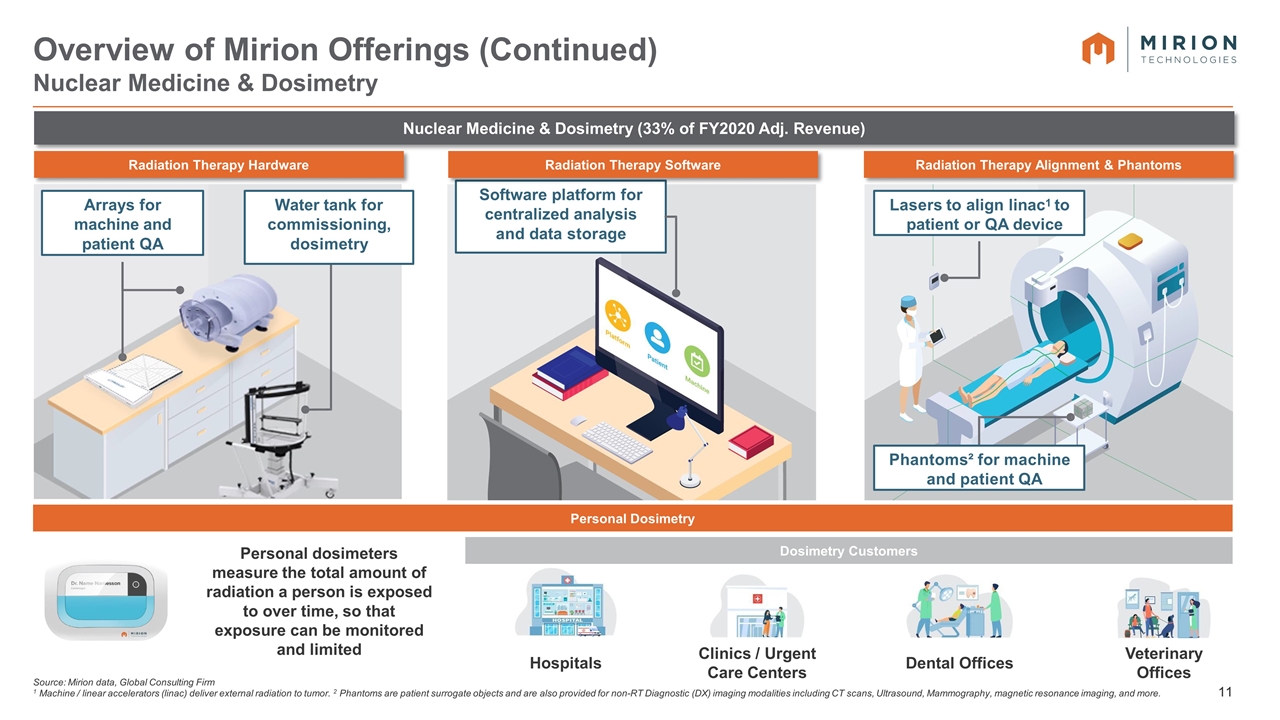
Source: Mirion data, Global Consulting Firm 1 Machine / linear accelerators (linac) deliver external radiation to tumor. 2 Phantoms are patient surrogate objects and are also provided for non-RT Diagnostic (DX) imaging modalities including CT scans, Ultrasound, Mammography, magnetic resonance imaging, and more. Overview of Mirion Offerings (Continued) Nuclear Medicine & Dosimetry Arrays for machine and patient QA Water tank for commissioning, dosimetry Radiation Therapy Hardware Software platform for centralized analysis and data storage Radiation Therapy Software Lasers to align linac1 to patient or QA device Phantoms² for machine and patient QA Radiation Therapy Alignment & Phantoms Personal Dosimetry Clinics / Urgent Care Centers Dental Offices Hospitals Veterinary Offices Dosimetry Customers Personal dosimeters measure the total amount of radiation a person is exposed to over time, so that exposure can be monitored and limited Nuclear Medicine & Dosimetry (33% of FY2020 Adj. Revenue)
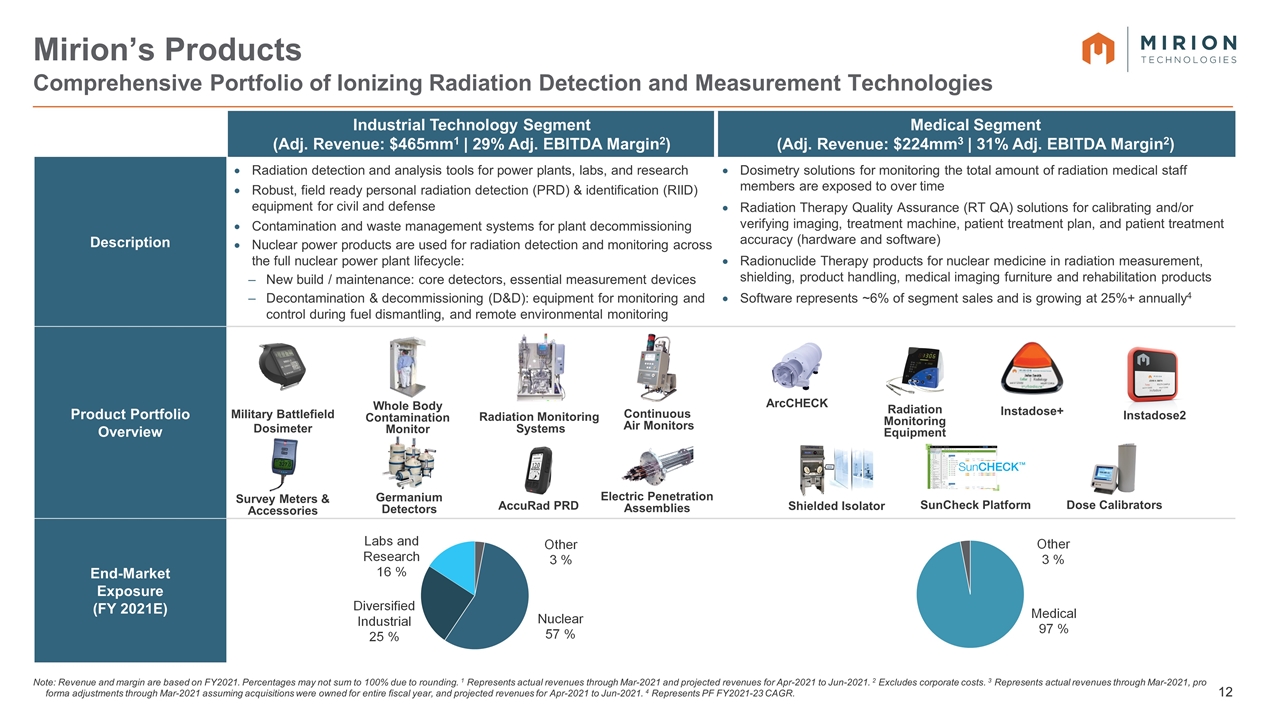
Mirion’s Products Comprehensive Portfolio of Ionizing Radiation Detection and Measurement Technologies Note: Revenue and margin are based on FY2021. Percentages may not sum to 100% due to rounding. 1 Represents actual revenues through Mar-2021 and projected revenues for Apr-2021 to Jun-2021. 2 Excludes corporate costs. 3 Represents actual revenues through Mar-2021, pro forma adjustments through Mar-2021 assuming acquisitions were owned for entire fiscal year, and projected revenues for Apr-2021 to Jun-2021. 4 Represents PF FY2021-23 CAGR. = Industrial Technology Segment (Adj. Revenue: $465mm1 | 29% Adj. EBITDA Margin2) Medical Segment (Adj. Revenue: $224mm3 | 31% Adj. EBITDA Margin2) Description Radiation detection and analysis tools for power plants, labs, and research Robust, field ready personal radiation detection (PRD) & identification (RIID) equipment for civil and defense Contamination and waste management systems for plant decommissioning Nuclear power products are used for radiation detection and monitoring across the full nuclear power plant lifecycle: New build / maintenance: core detectors, essential measurement devices Decontamination & decommissioning (D&D): equipment for monitoring and control during fuel dismantling, and remote environmental monitoring Dosimetry solutions for monitoring the total amount of radiation medical staff members are exposed to over time Radiation Therapy Quality Assurance (RT QA) solutions for calibrating and/or verifying imaging, treatment machine, patient treatment plan, and patient treatment accuracy (hardware and software) Radionuclide Therapy products for nuclear medicine in radiation measurement, shielding, product handling, medical imaging furniture and rehabilitation products Software represents ~6% of segment sales and is growing at 25%+ annually4 Product Portfolio Overview End-Market Exposure (FY 2021E) Shielded Isolator Radiation Monitoring Equipment ArcCHECK Instadose+ Whole Body Contamination Monitor Germanium Detectors Radiation Monitoring Systems Electric Penetration Assemblies SunCheck Platform Survey Meters & Accessories Military Battlefield Dosimeter AccuRad PRD Continuous Air Monitors Dose Calibrators Instadose2
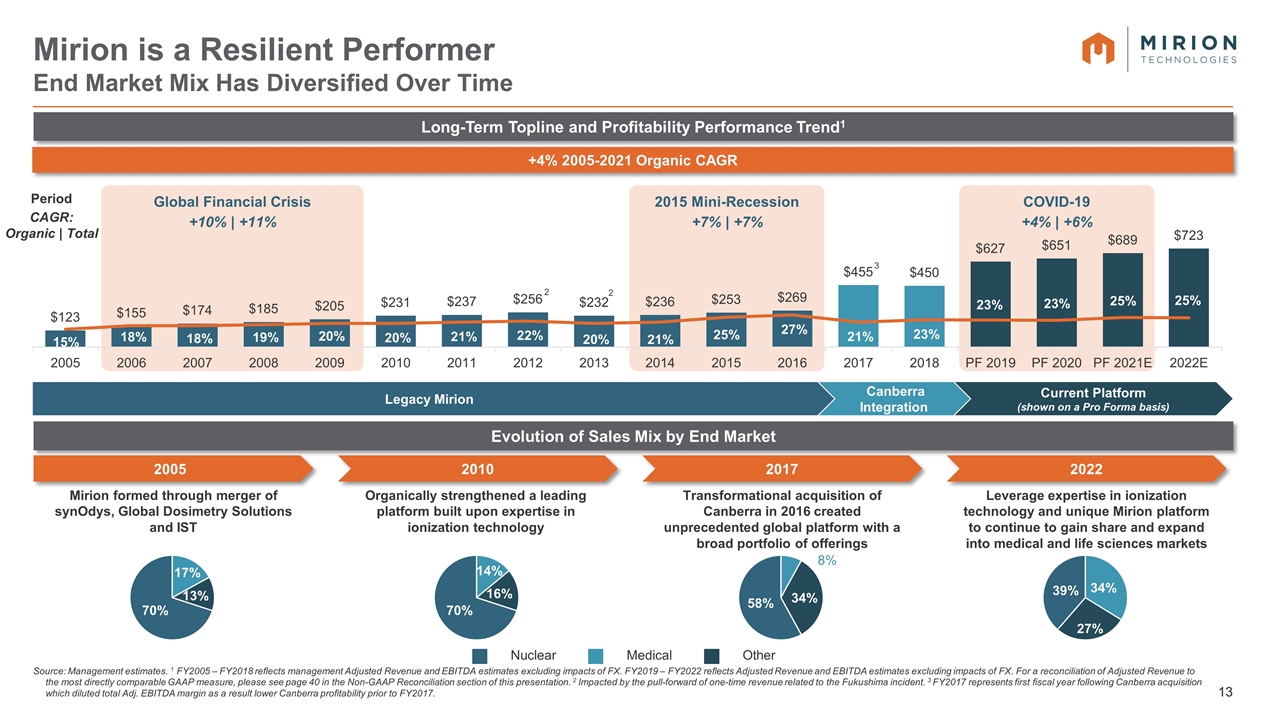
2015 Mini-Recession +7% | +7% Global Financial Crisis +10% | +11% COVID-19 +4% | +6% Period CAGR: Organic | Total Source: Management estimates. 1 FY2005 – FY2018 reflects management Adjusted Revenue and EBITDA estimates excluding impacts of FX. FY2019 – FY2022 reflects Adjusted Revenue and EBITDA estimates excluding impacts of FX. For a reconciliation of Adjusted Revenue to the most directly comparable GAAP measure, please see page 40 in the Non-GAAP Reconciliation section of this presentation. 2 Impacted by the pull-forward of one-time revenue related to the Fukushima incident. 3 FY2017 represents first fiscal year following Canberra acquisition which diluted total Adj. EBITDA margin as a result lower Canberra profitability prior to FY2017. Mirion is a Resilient Performer End Market Mix Has Diversified Over Time 2005 Mirion formed through merger of synOdys, Global Dosimetry Solutions and IST Current Platform (shown on a Pro Forma basis) Canberra Integration Legacy Mirion Long-Term Topline and Profitability Performance Trend1 +4% 2005-2021 Organic CAGR Evolution of Sales Mix by End Market 2010 Organically strengthened a leading platform built upon expertise in ionization technology 2017 Transformational acquisition of Canberra in 2016 created unprecedented global platform with a broad portfolio of offerings 2022 Leverage expertise in ionization technology and unique Mirion platform to continue to gain share and expand into medical and life sciences markets Nuclear Medical Other 2005-2016: Margins come from Matrix CIM Revenue comes from: \\firmwide.corp.gs.com\ibdroot\projects\IBD-NY\gumdrops2020A\671701_1\02. Received\Copy of Mirion LT Revenue Trend_2020.04.08.xlsx 2017-2022: \\firmwide.corp.gs.com\ibdroot\projects\IBD-NY\gumdrops2020A\671701_1\02. Received\Mirion CY and FY Financials_2021.04.02 vSS.xlsx 2 3 2

II. Key Investment Highlights
Key Investment Highlights Large, stable and growing end markets Leading competitive position and longstanding customer relationships Growth profile augmented by attractive M&A pipeline Multiple paths for continued outperformance Best-in-class management team 1 6 5 4 2 Strong, resilient business model with strong organic growth 3
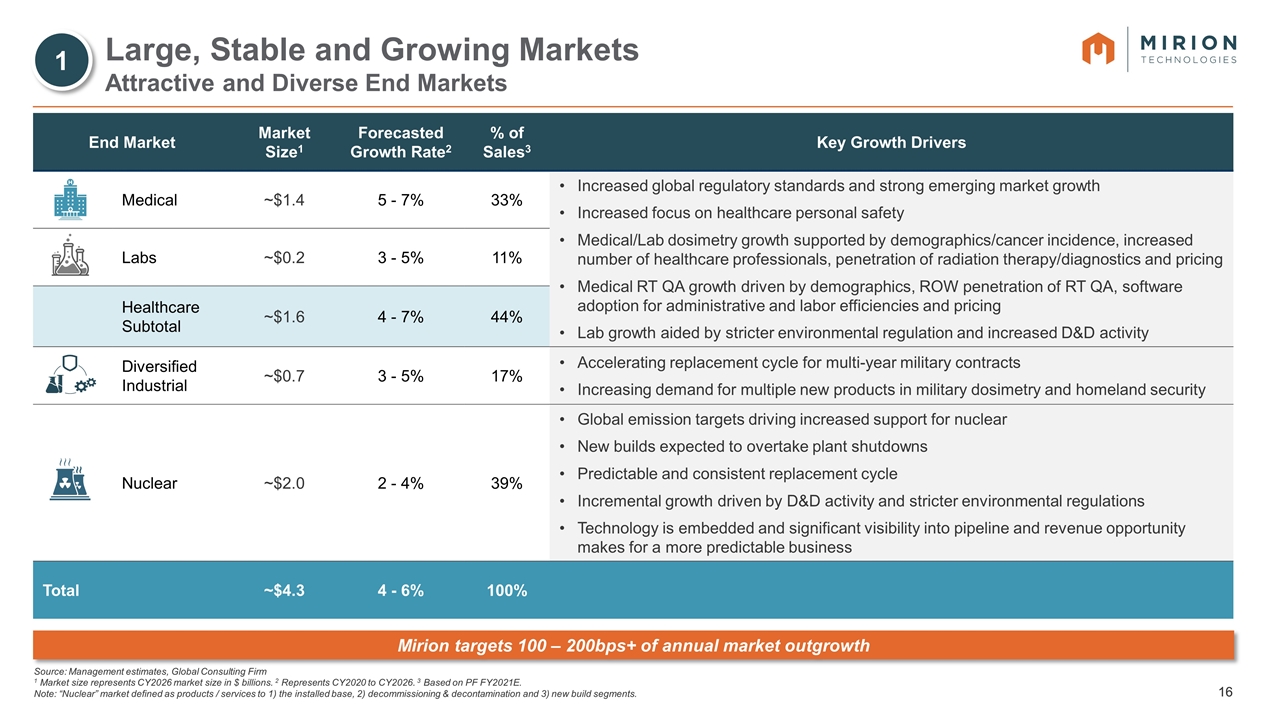
Source: Management estimates, Global Consulting Firm 1 Market size represents CY2026 market size in $ billions. 2 Represents CY2020 to CY2026. 3 Based on PF FY2021E. Note: “Nuclear” market defined as products / services to 1) the installed base, 2) decommissioning & decontamination and 3) new build segments. Large, Stable and Growing Markets Attractive and Diverse End Markets End Market Market Size1 Forecasted Growth Rate2 % of Sales3 Key Growth Drivers Medical ~$1.4 5 - 7% 33% Increased global regulatory standards and strong emerging market growth Increased focus on healthcare personal safety Medical/Lab dosimetry growth supported by demographics/cancer incidence, increased number of healthcare professionals, penetration of radiation therapy/diagnostics and pricing Medical RT QA growth driven by demographics, ROW penetration of RT QA, software adoption for administrative and labor efficiencies and pricing Lab growth aided by stricter environmental regulation and increased D&D activity Labs ~$0.2 3 - 5% 11% Healthcare Subtotal ~$1.6 4 - 7% 44% Diversified Industrial ~$0.7 3 - 5% 17% Accelerating replacement cycle for multi-year military contracts Increasing demand for multiple new products in military dosimetry and homeland security Nuclear ~$2.0 2 - 4% 39% Global emission targets driving increased support for nuclear New builds expected to overtake plant shutdowns Predictable and consistent replacement cycle Incremental growth driven by D&D activity and stricter environmental regulations Technology is embedded and significant visibility into pipeline and revenue opportunity makes for a more predictable business Total ~$4.3 4 - 6% 100% Mirion targets 100 – 200bps+ of annual market outgrowth 1
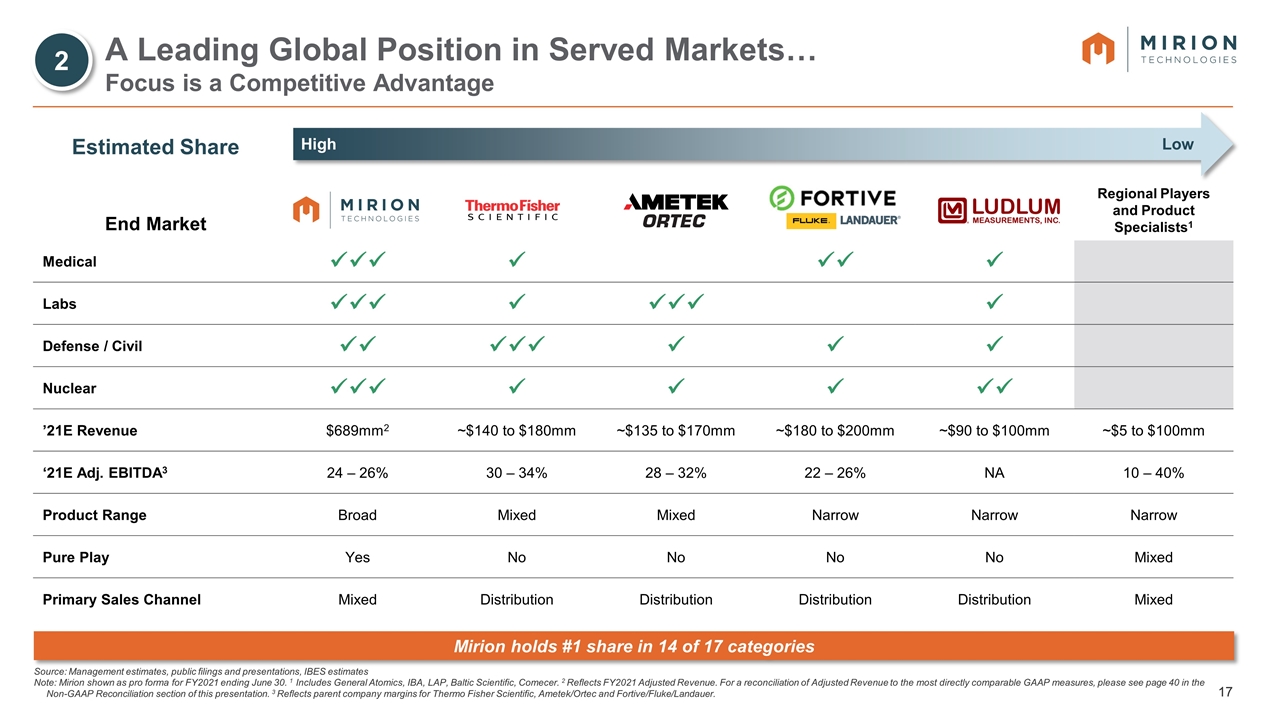
Estimated Share End Market Regional Players and Product Specialists1 Medical üüü ü üü ü Labs üüü ü üüü ü Defense / Civil üü üüü ü ü ü Nuclear üüü ü ü ü üü ’21E Revenue $689mm2 ~$140 to $180mm ~$135 to $170mm ~$180 to $200mm ~$90 to $100mm ~$5 to $100mm ‘21E Adj. EBITDA3 24 – 26% 30 – 34% 28 – 32% 22 – 26% NA 10 – 40% Product Range Broad Mixed Mixed Narrow Narrow Narrow Pure Play Yes No No No No Mixed Primary Sales Channel Mixed Distribution Distribution Distribution Distribution Mixed A Leading Global Position in Served Markets… Focus is a Competitive Advantage High Low Source: Management estimates, public filings and presentations, IBES estimates Note: Mirion shown as pro forma for FY2021 ending June 30. 1 Includes General Atomics, IBA, LAP, Baltic Scientific, Comecer. 2 Reflects FY2021 Adjusted Revenue. For a reconciliation of Adjusted Revenue to the most directly comparable GAAP measures, please see page 40 in the Non-GAAP Reconciliation section of this presentation. 3 Reflects parent company margins for Thermo Fisher Scientific, Ametek/Ortec and Fortive/Fluke/Landauer. Mirion holds #1 share in 14 of 17 categories 2
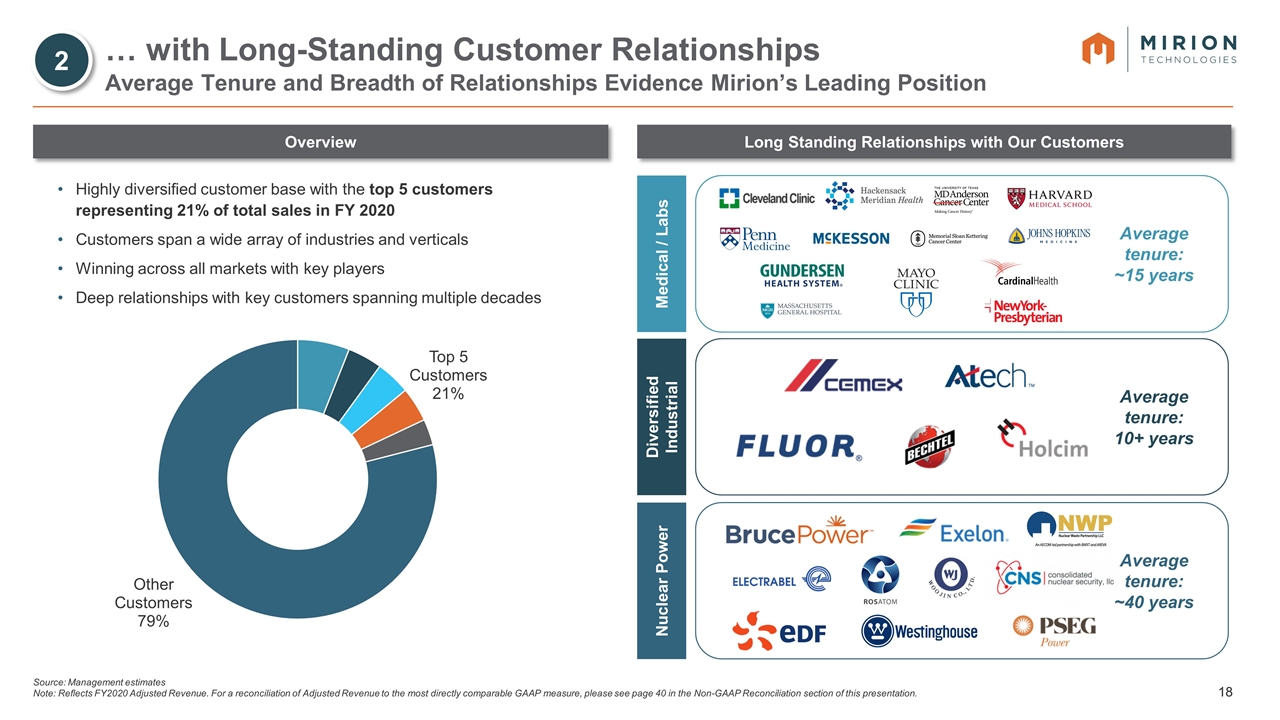
Source: Management estimates Note: Reflects FY2020 Adjusted Revenue. For a reconciliation of Adjusted Revenue to the most directly comparable GAAP measure, please see page 40 in the Non-GAAP Reconciliation section of this presentation. … with Long-Standing Customer Relationships Average Tenure and Breadth of Relationships Evidence Mirion’s Leading Position Highly diversified customer base with the top 5 customers representing 21% of total sales in FY 2020 Customers span a wide array of industries and verticals Winning across all markets with key players Deep relationships with key customers spanning multiple decades Overview Long Standing Relationships with Our Customers Average tenure: ~15 years Medical / Labs Average tenure: 10+ years Diversified Industrial Average tenure: ~40 years Nuclear Power 2
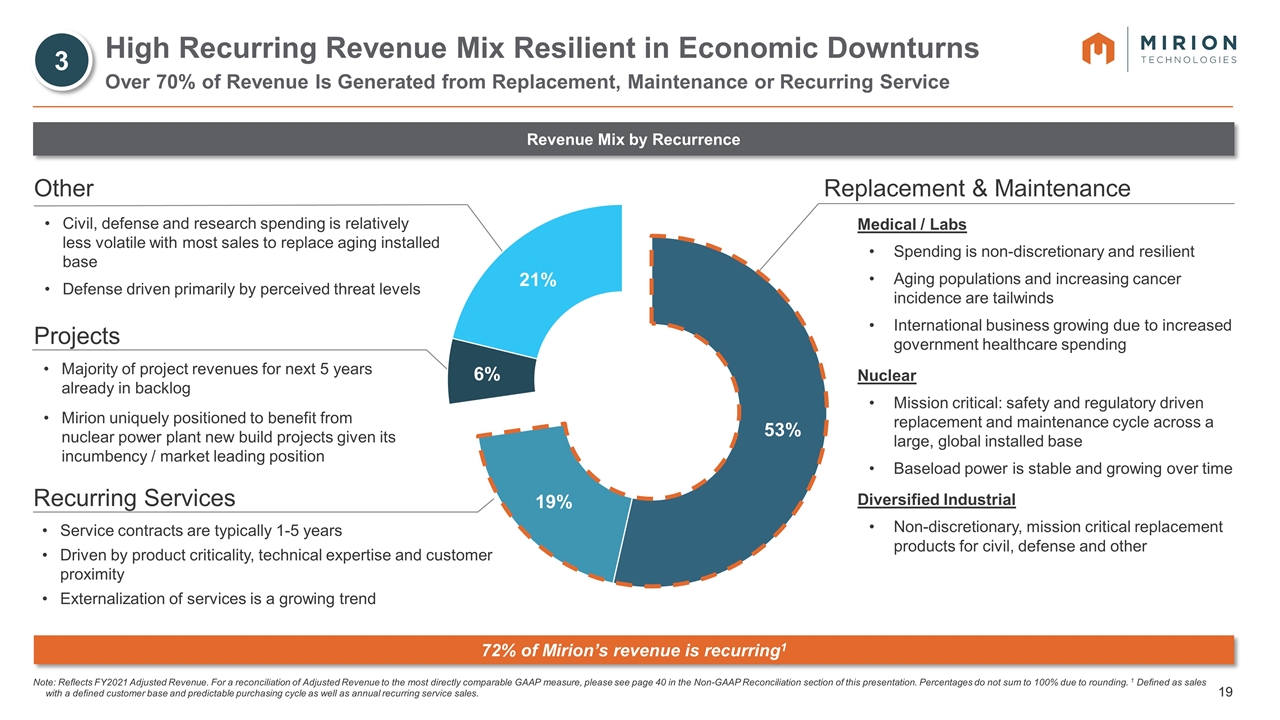
Other Projects Majority of project revenues for next 5 years already in backlog Mirion uniquely positioned to benefit from nuclear power plant new build projects given its incumbency / market leading position Recurring Services Service contracts are typically 1-5 years Driven by product criticality, technical expertise and customer proximity Externalization of services is a growing trend Civil, defense and research spending is relatively less volatile with most sales to replace aging installed base Defense driven primarily by perceived threat levels Medical / Labs Spending is non-discretionary and resilient Aging populations and increasing cancer incidence are tailwinds International business growing due to increased government healthcare spending Nuclear Mission critical: safety and regulatory driven replacement and maintenance cycle across a large, global installed base Baseload power is stable and growing over time Diversified Industrial Non-discretionary, mission critical replacement products for civil, defense and other Note: Reflects FY2021 Adjusted Revenue. For a reconciliation of Adjusted Revenue to the most directly comparable GAAP measure, please see page 40 in the Non-GAAP Reconciliation section of this presentation. Percentages do not sum to 100% due to rounding. 1 Defined as sales with a defined customer base and predictable purchasing cycle as well as annual recurring service sales. High Recurring Revenue Mix Resilient in Economic Downturns Over 70% of Revenue Is Generated from Replacement, Maintenance or Recurring Service Revenue Mix by Recurrence Replacement & Maintenance 72% of Mirion’s revenue is recurring1 3
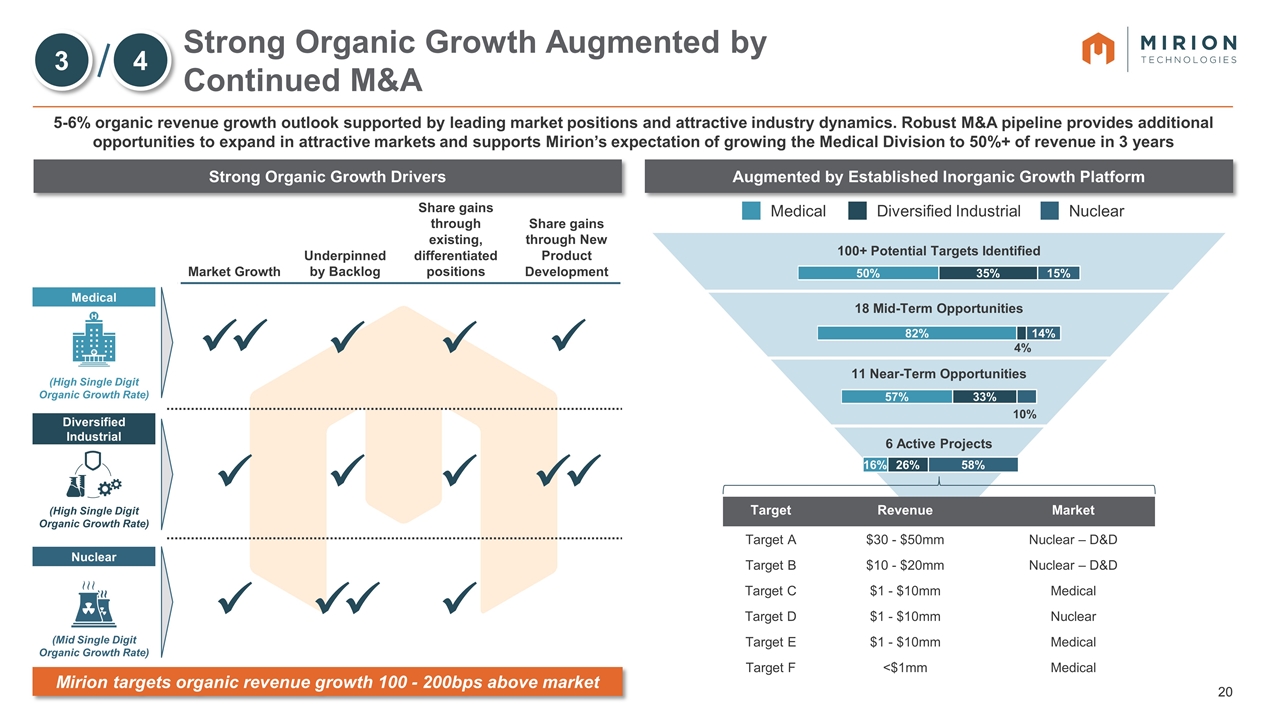
Market Growth Underpinned by Backlog Share gains through existing, differentiated positions Share gains through New Product Development Strong Organic Growth Augmented by Continued M&A 5-6% organic revenue growth outlook supported by leading market positions and attractive industry dynamics. Robust M&A pipeline provides additional opportunities to expand in attractive markets and supports Mirion’s expectation of growing the Medical Division to 50%+ of revenue in 3 years Strong Organic Growth Drivers Augmented by Established Inorganic Growth Platform Medical Nuclear Diversified Industrial (High Single Digit Organic Growth Rate) (Mid Single Digit Organic Growth Rate) (High Single Digit Organic Growth Rate) ü ü ü ü ü ü ü ü ü ü ü ü ü ü 100+ Potential Targets Identified 18 Mid-Term Opportunities 11 Near-Term Opportunities 6 Active Projects Medical Diversified Industrial Nuclear Mirion targets organic revenue growth 100 - 200bps above market 3 4 Target Revenue Market Target A $30 - $50mm Nuclear – D&D Target B $10 - $20mm Nuclear – D&D Target C $1 - $10mm Medical Target D $1 - $10mm Nuclear Target E $1 - $10mm Medical Target F <$1mm Medical
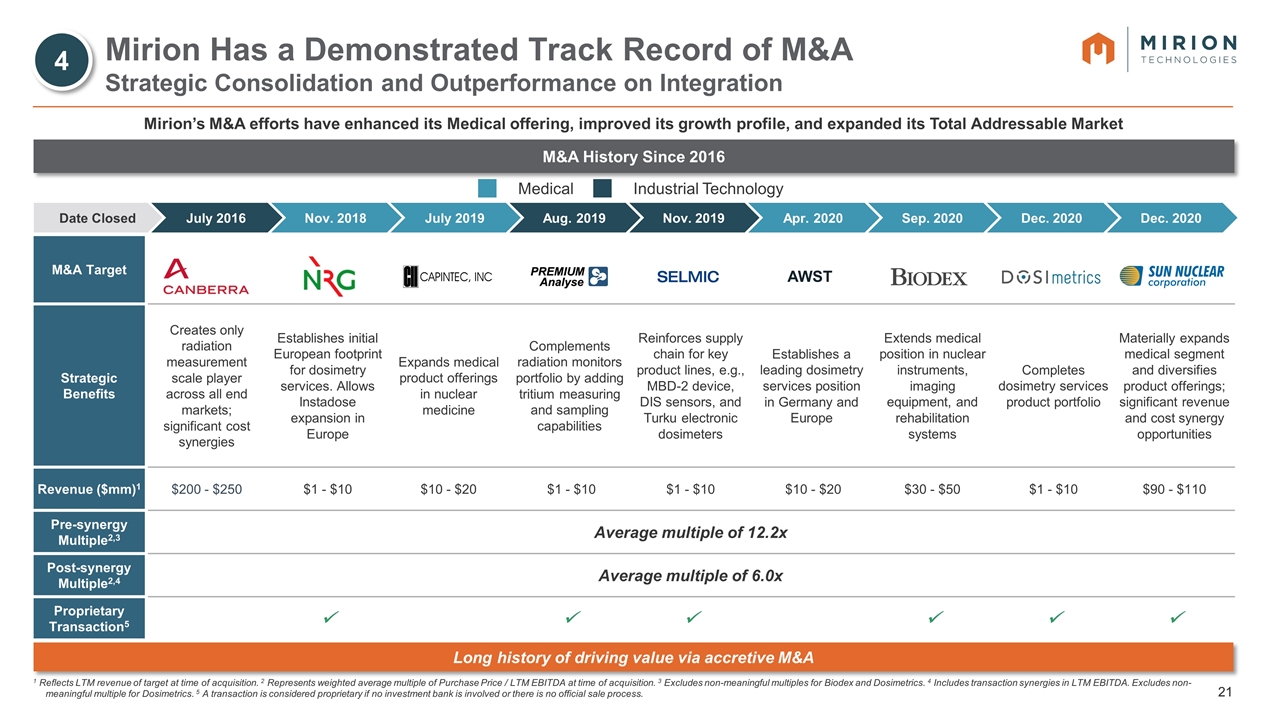
M&A Target Strategic Benefits Creates only radiation measurement scale player across all end markets; significant cost synergies Establishes initial European footprint for dosimetry services. Allows Instadose expansion in Europe Expands medical product offerings in nuclear medicine Complements radiation monitors portfolio by adding tritium measuring and sampling capabilities Reinforces supply chain for key product lines, e.g., MBD-2 device, DIS sensors, and Turku electronic dosimeters Establishes a leading dosimetry services position in Germany and Europe Extends medical position in nuclear instruments, imaging equipment, and rehabilitation systems Completes dosimetry services product portfolio Materially expands medical segment and diversifies product offerings; significant revenue and cost synergy opportunities Revenue ($mm)1 $200 - $250 $1 - $10 $10 - $20 $1 - $10 $1 - $10 $10 - $20 $30 - $50 $1 - $10 $90 - $110 Pre-synergy Multiple2,3 Average multiple of 12.2x Post-synergy Multiple2,4 Average multiple of 6.0x Proprietary Transaction5 ü ü ü ü ü ü Medical Industrial Technology Mirion Has a Demonstrated Track Record of M&A Strategic Consolidation and Outperformance on Integration 1 Reflects LTM revenue of target at time of acquisition. 2 Represents weighted average multiple of Purchase Price / LTM EBITDA at time of acquisition. 3 Excludes non-meaningful multiples for Biodex and Dosimetrics. 4 Includes transaction synergies in LTM EBITDA. Excludes non-meaningful multiple for Dosimetrics. 5 A transaction is considered proprietary if no investment bank is involved or there is no official sale process. Mirion’s M&A efforts have enhanced its Medical offering, improved its growth profile, and expanded its Total Addressable Market M&A History Since 2016 AWST Long history of driving value via accretive M&A Date Closed July 2019 Nov. 2019 Aug. 2019 Sep. 2020 Apr. 2020 Dec. 2020 Dec. 2020 Nov. 2018 July 2016 4
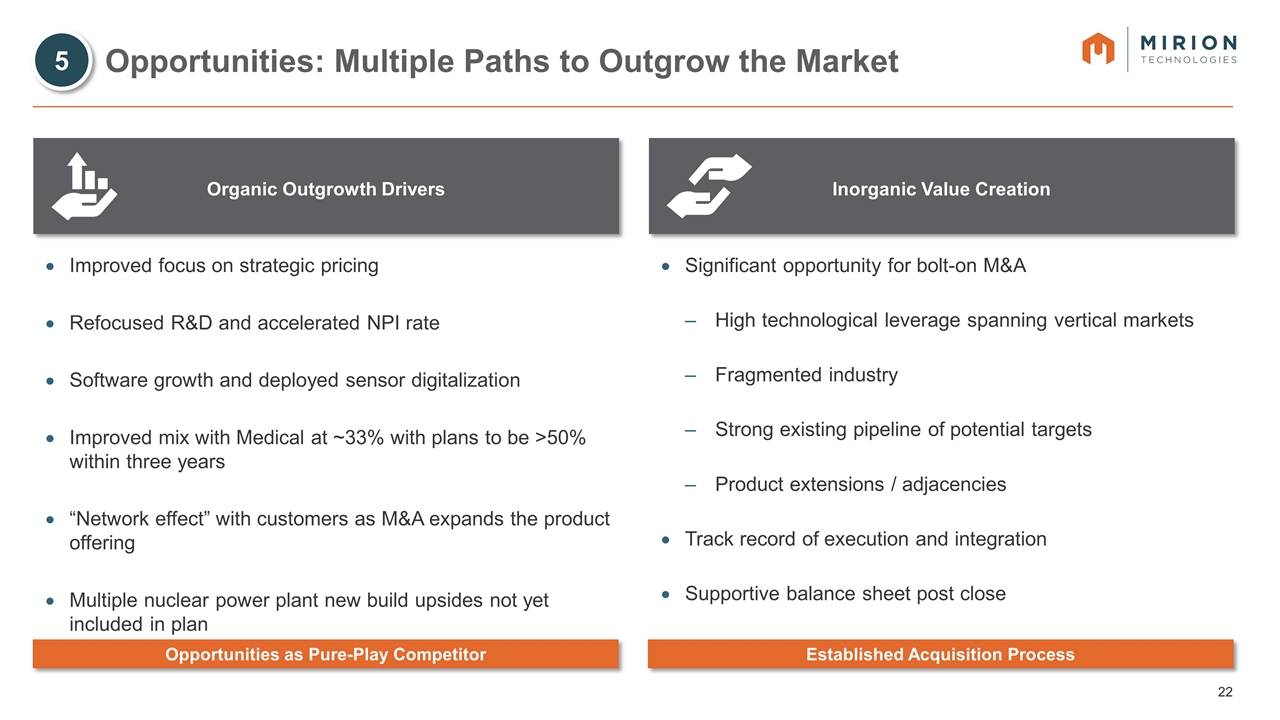
Organic Outgrowth Drivers Inorganic Value Creation Opportunities: Multiple Paths to Outgrow the Market Improved focus on strategic pricing Refocused R&D and accelerated NPI rate Software growth and deployed sensor digitalization Improved mix with Medical at ~33% with plans to be >50% within three years “Network effect” with customers as M&A expands the product offering Multiple nuclear power plant new build upsides not yet included in plan Significant opportunity for bolt-on M&A High technological leverage spanning vertical markets Fragmented industry Strong existing pipeline of potential targets Product extensions / adjacencies Track record of execution and integration Supportive balance sheet post close 5 Opportunities as Pure-Play Competitor Established Acquisition Process
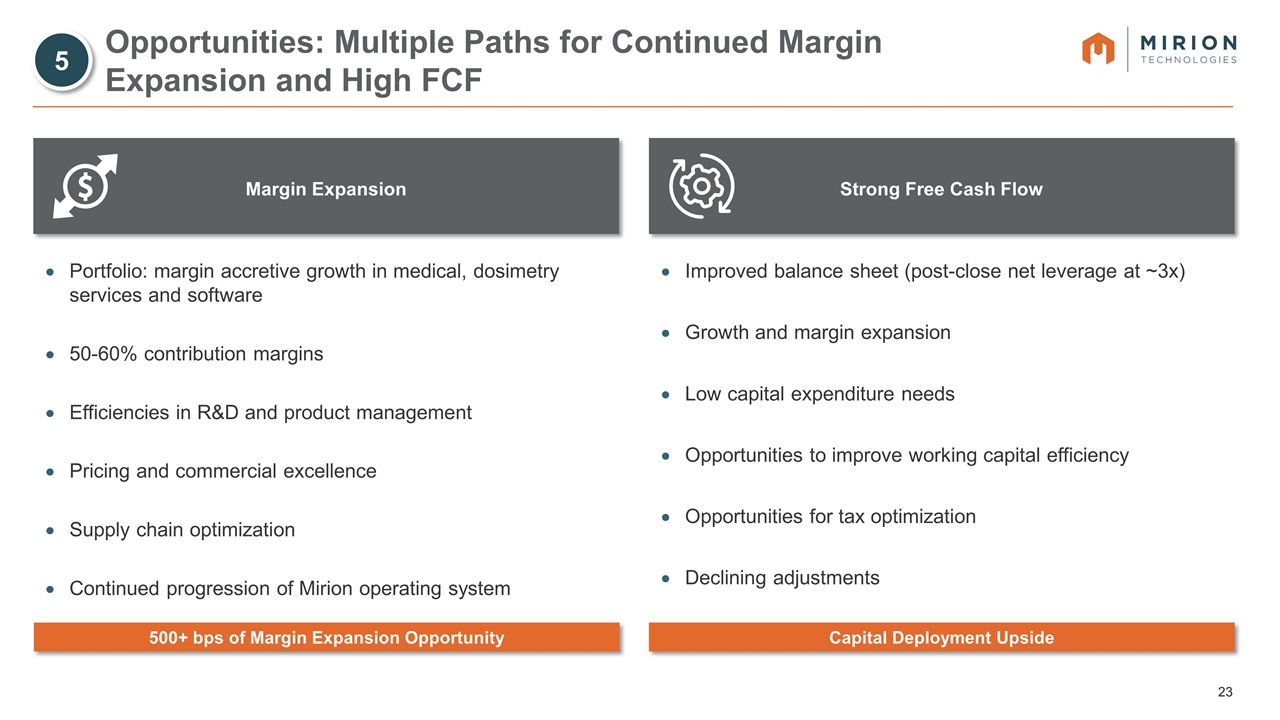
Margin Expansion Strong Free Cash Flow Opportunities: Multiple Paths for Continued Margin Expansion and High FCF Portfolio: margin accretive growth in medical, dosimetry services and software 50-60% contribution margins Efficiencies in R&D and product management Pricing and commercial excellence Supply chain optimization Continued progression of Mirion operating system Improved balance sheet (post-close net leverage at ~3x) Growth and margin expansion Low capital expenditure needs Opportunities to improve working capital efficiency Opportunities for tax optimization Declining adjustments 500+ bps of Margin Expansion Opportunity Capital Deployment Upside 5
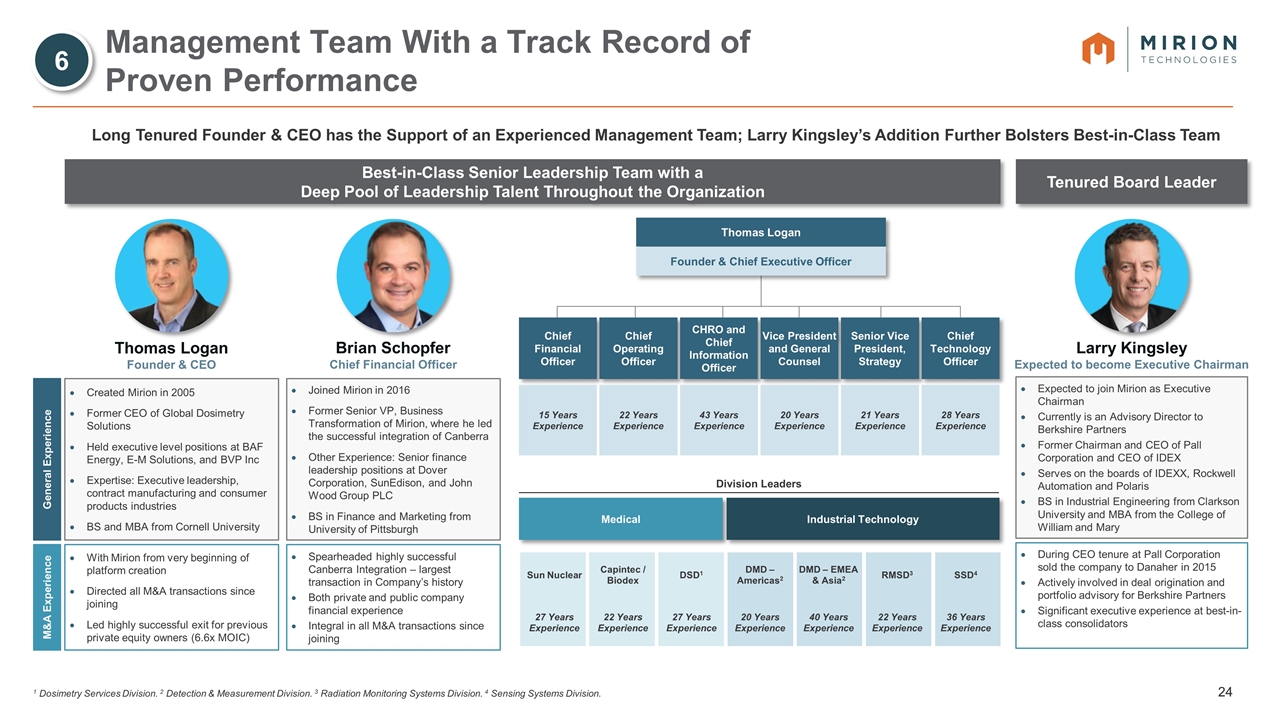
Sun Nuclear Capintec / Biodex DSD1 DMD – Americas2 DMD – EMEA & Asia2 RMSD3 SSD4 27 Years Experience 22 Years Experience 27 Years Experience 20 Years Experience 40 Years Experience 22 Years Experience 36 Years Experience Management Team With a Track Record of Proven Performance Long Tenured Founder & CEO has the Support of an Experienced Management Team; Larry Kingsley’s Addition Further Bolsters Best-in-Class Team M&A Experience General Experience Chief Financial Officer Chief Operating Officer CHRO and Chief Information Officer Vice President and General Counsel Senior Vice President, Strategy Chief Technology Officer 15 Years Experience 43 Years Experience 20 Years Experience 21 Years Experience 28 Years Experience Thomas Logan Founder & Chief Executive Officer 22 Years Experience Medical Industrial Technology Division Leaders Best-in-Class Senior Leadership Team with a Deep Pool of Leadership Talent Throughout the Organization Thomas Logan Founder & CEO Created Mirion in 2005 Former CEO of Global Dosimetry Solutions Held executive level positions at BAF Energy, E-M Solutions, and BVP Inc Expertise: Executive leadership, contract manufacturing and consumer products industries BS and MBA from Cornell University With Mirion from very beginning of platform creation Directed all M&A transactions since joining Led highly successful exit for previous private equity owners (6.6x MOIC) Brian Schopfer Chief Financial Officer Joined Mirion in 2016 Former Senior VP, Business Transformation of Mirion, where he led the successful integration of Canberra Other Experience: Senior finance leadership positions at Dover Corporation, SunEdison, and John Wood Group PLC BS in Finance and Marketing from University of Pittsburgh Spearheaded highly successful Canberra Integration – largest transaction in Company’s history Both private and public company financial experience Integral in all M&A transactions since joining Expected to join Mirion as Executive Chairman Currently is an Advisory Director to Berkshire Partners Former Chairman and CEO of Pall Corporation and CEO of IDEX Serves on the boards of IDEXX, Rockwell Automation and Polaris BS in Industrial Engineering from Clarkson University and MBA from the College of William and Mary During CEO tenure at Pall Corporation sold the company to Danaher in 2015 Actively involved in deal origination and portfolio advisory for Berkshire Partners Significant executive experience at best-in-class consolidators Larry Kingsley Expected to become Executive Chairman Tenured Board Leader 6 1 Dosimetry Services Division. 2 Detection & Measurement Division. 3 Radiation Monitoring Systems Division. 4 Sensing Systems Division.

III. Financial Overview
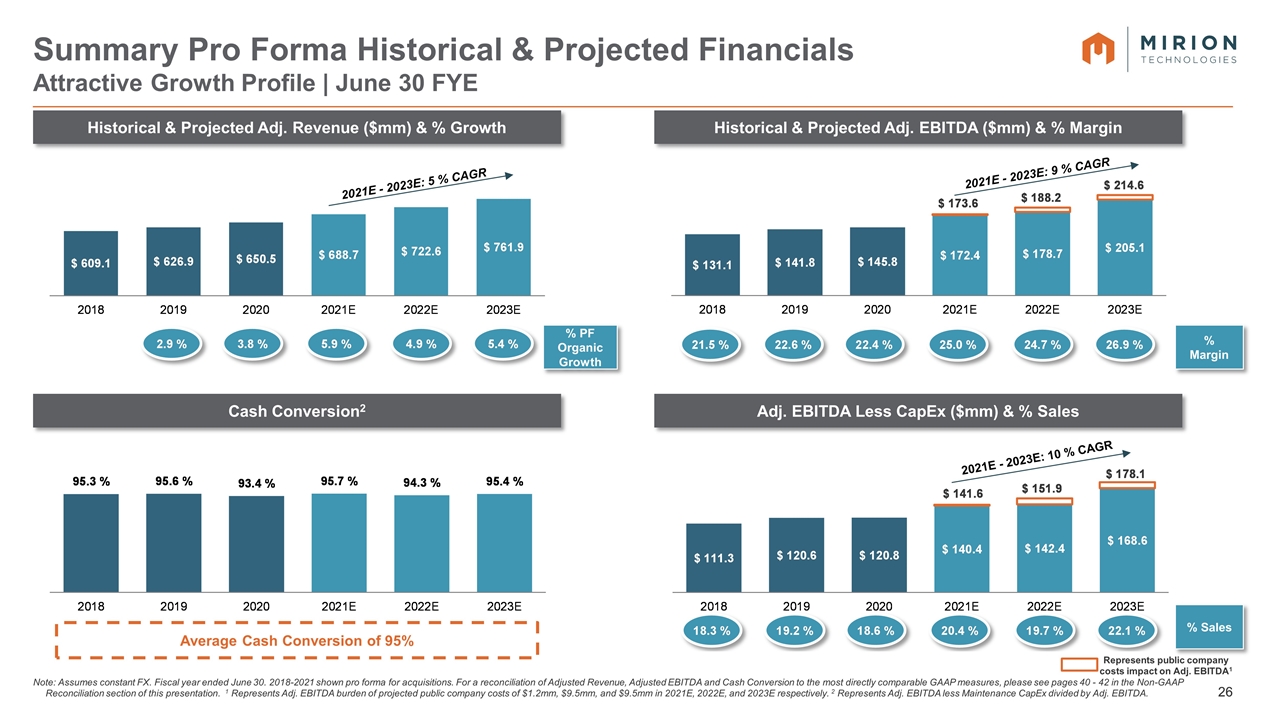
Note: Assumes constant FX. Fiscal year ended June 30. 2018-2021 shown pro forma for acquisitions. For a reconciliation of Adjusted Revenue, Adjusted EBITDA and Cash Conversion to the most directly comparable GAAP measures, please see pages 40 - 42 in the Non-GAAP Reconciliation section of this presentation. 1 Represents Adj. EBITDA burden of projected public company costs of $1.2mm, $9.5mm, and $9.5mm in 2021E, 2022E, and 2023E respectively. 2 Represents Adj. EBITDA less Maintenance CapEx divided by Adj. EBITDA. Summary Pro Forma Historical & Projected Financials Attractive Growth Profile | June 30 FYE Historical & Projected Adj. Revenue ($mm) & % Growth Historical & Projected Adj. EBITDA ($mm) & % Margin Adj. EBITDA Less CapEx ($mm) & % Sales % Margin % Sales % PF Organic Growth Represents public company costs impact on Adj. EBITDA1 Cash Conversion2 Average Cash Conversion of 95%
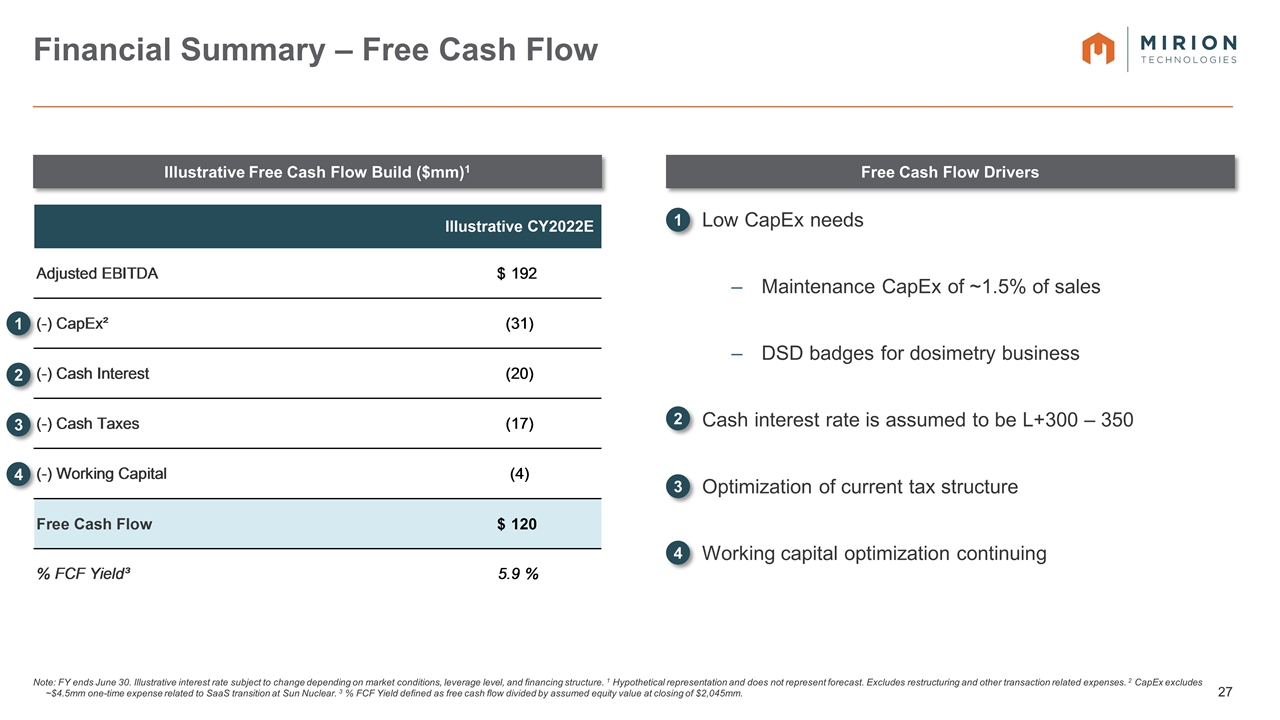
Low CapEx needs Maintenance CapEx of ~1.5% of sales DSD badges for dosimetry business Cash interest rate is assumed to be L+300 – 350 Optimization of current tax structure Working capital optimization continuing Note: FY ends June 30. Illustrative interest rate subject to change depending on market conditions, leverage level, and financing structure. 1 Hypothetical representation and does not represent forecast. Excludes restructuring and other transaction related expenses. 2 CapEx excludes ~$4.5mm one-time expense related to SaaS transition at Sun Nuclear. 3 % FCF Yield defined as free cash flow divided by assumed equity value at closing of $2,045mm. Financial Summary – Free Cash Flow Illustrative Free Cash Flow Build ($mm)1 Free Cash Flow Drivers 1 2 3 4 1 2 3 4
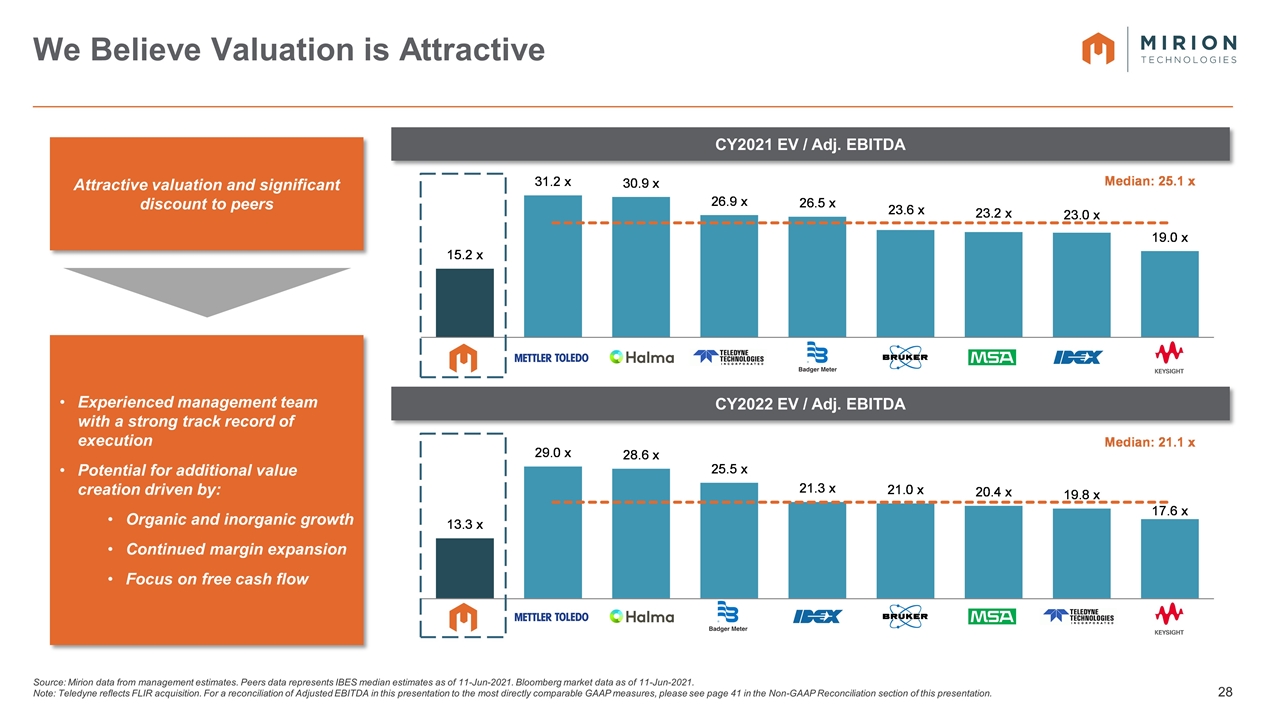
Attractive valuation and significant discount to peers Experienced management team with a strong track record of execution Potential for additional value creation driven by: Organic and inorganic growth Continued margin expansion Focus on free cash flow Source: Mirion data from management estimates. Peers data represents IBES median estimates as of 11-Jun-2021. Bloomberg market data as of 11-Jun-2021. Note: Teledyne reflects FLIR acquisition. For a reconciliation of Adjusted EBITDA in this presentation to the most directly comparable GAAP measures, please see page 41 in the Non-GAAP Reconciliation section of this presentation. We Believe Valuation is Attractive CY2021 EV / Adj. EBITDA CY2022 EV / Adj. EBITDA
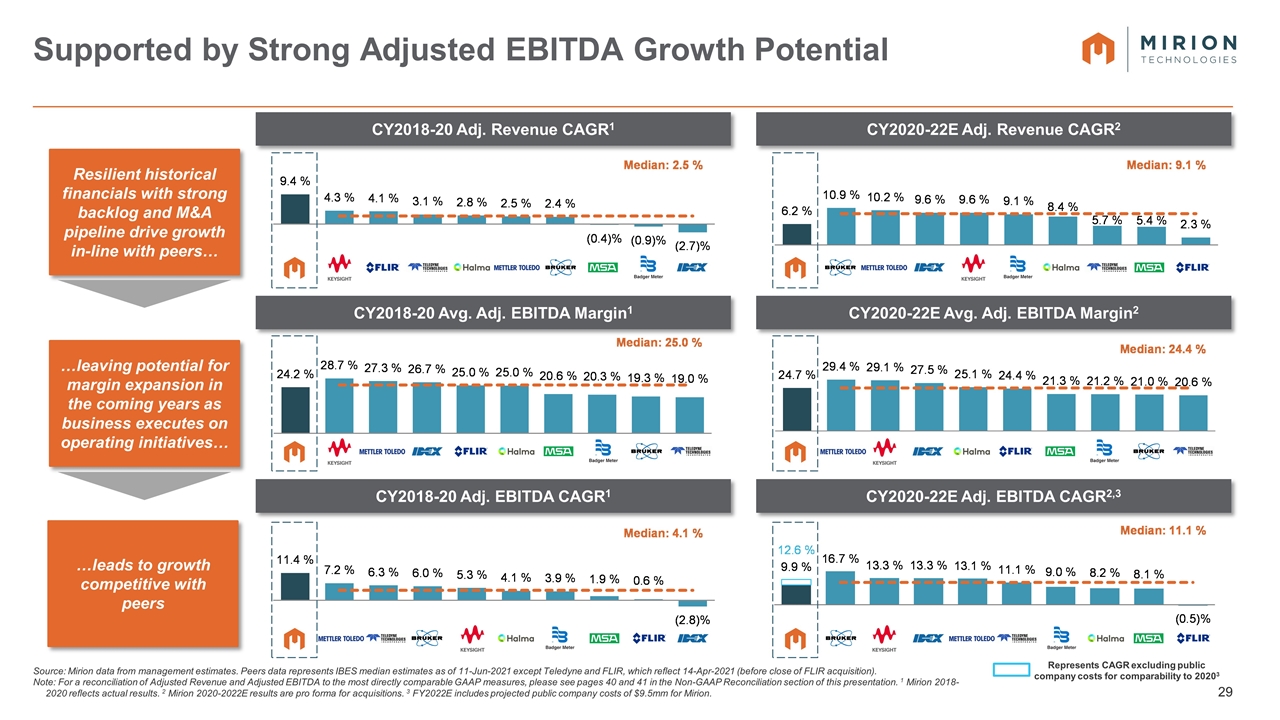
Supported by Strong Adjusted EBITDA Growth Potential Source: Mirion data from management estimates. Peers data represents IBES median estimates as of 11-Jun-2021 except Teledyne and FLIR, which reflect 14-Apr-2021 (before close of FLIR acquisition). Note: For a reconciliation of Adjusted Revenue and Adjusted EBITDA to the most directly comparable GAAP measures, please see pages 40 and 41 in the Non-GAAP Reconciliation section of this presentation. 1 Mirion 2018-2020 reflects actual results. 2 Mirion 2020-2022E results are pro forma for acquisitions. 3 FY2022E includes projected public company costs of $9.5mm for Mirion. CY2020-22E Adj. Revenue CAGR2 CY2018-20 Adj. Revenue CAGR1 CY2020-22E Avg. Adj. EBITDA Margin2 CY2018-20 Avg. Adj. EBITDA Margin1 CY2020-22E Adj. EBITDA CAGR2,3 CY2018-20 Adj. EBITDA CAGR1 Resilient historical financials with strong backlog and M&A pipeline drive growth in-line with peers… …leads to growth competitive with peers …leaving potential for margin expansion in the coming years as business executes on operating initiatives… Represents CAGR excluding public company costs for comparability to 20203
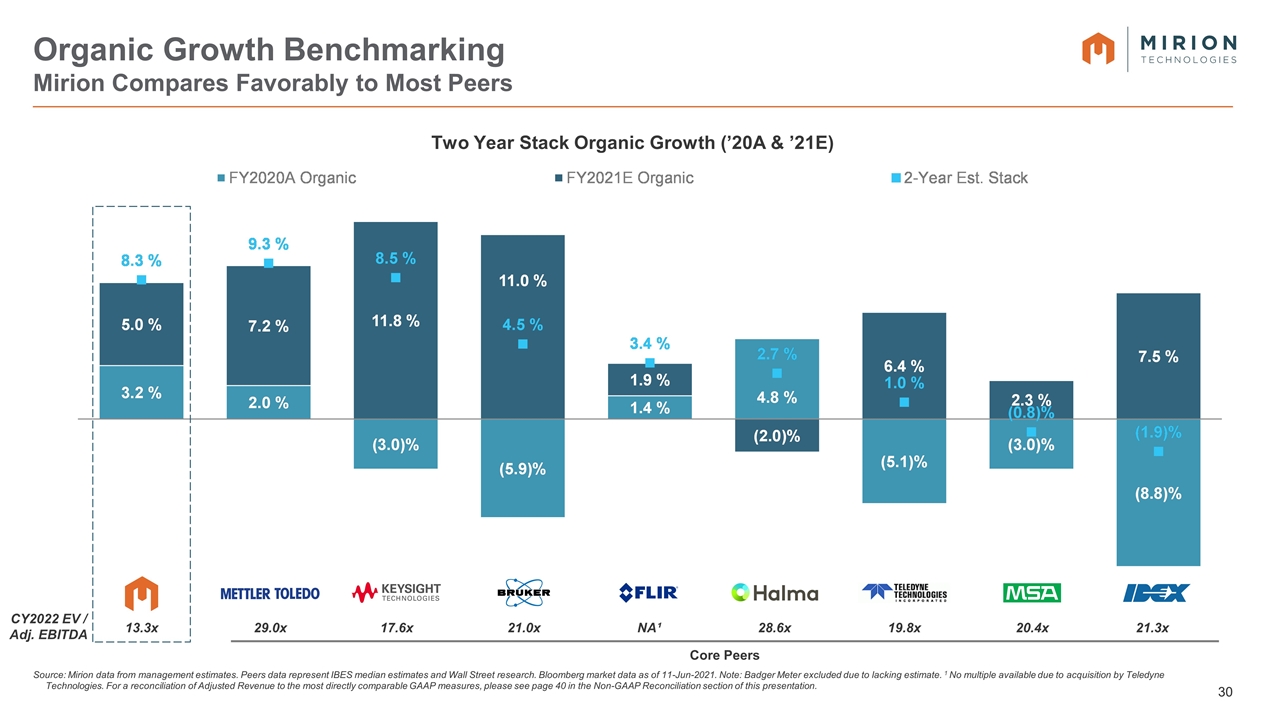
Organic Growth Benchmarking Mirion Compares Favorably to Most Peers Source: Mirion data from management estimates. Peers data represent IBES median estimates and Wall Street research. Bloomberg market data as of 11-Jun-2021. Note: Badger Meter excluded due to lacking estimate. 1 No multiple available due to acquisition by Teledyne Technologies. For a reconciliation of Adjusted Revenue to the most directly comparable GAAP measures, please see page 40 in the Non-GAAP Reconciliation section of this presentation. Core Peers Two Year Stack Organic Growth (’20A & ’21E) CY2022 EV / Adj. EBITDA 13.3x 29.0x 17.6x 21.0x NA¹ 28.6x 19.8x 21.3x 20.4x

Appendix
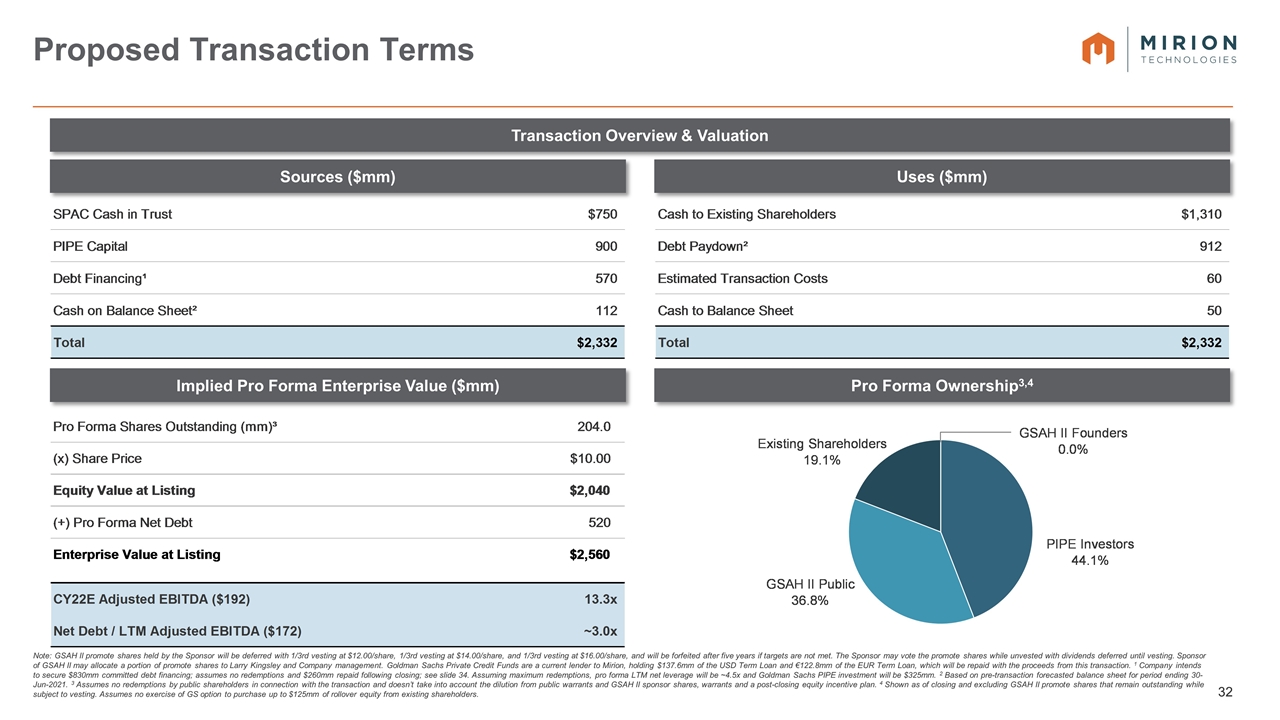
Note: GSAH II promote shares held by the Sponsor will be deferred with 1/3rd vesting at $12.00/share, 1/3rd vesting at $14.00/share, and 1/3rd vesting at $16.00/share, and will be forfeited after five years if targets are not met. The Sponsor may vote the promote shares while unvested with dividends deferred until vesting. Sponsor of GSAH II may allocate a portion of promote shares to Larry Kingsley and Company management. Goldman Sachs Private Credit Funds are a current lender to Mirion, holding $137.6mm of the USD Term Loan and €122.8mm of the EUR Term Loan, which will be repaid with the proceeds from this transaction. 1 Company intends to secure $830mm committed debt financing; assumes no redemptions and $260mm repaid following closing; see slide 34. Assuming maximum redemptions, pro forma LTM net leverage will be ~4.5x and Goldman Sachs PIPE investment will be $325mm. 2 Based on pre-transaction forecasted balance sheet for period ending 30-Jun-2021. 3 Assumes no redemptions by public shareholders in connection with the transaction and doesn’t take into account the dilution from public warrants and GSAH II sponsor shares, warrants and a post-closing equity incentive plan. 4 Shown as of closing and excluding GSAH II promote shares that remain outstanding while subject to vesting. Assumes no exercise of GS option to purchase up to $125mm of rollover equity from existing shareholders. Proposed Transaction Terms Sources ($mm) Transaction Overview & Valuation Implied Pro Forma Enterprise Value ($mm) Uses ($mm) Pro Forma Ownership3,4
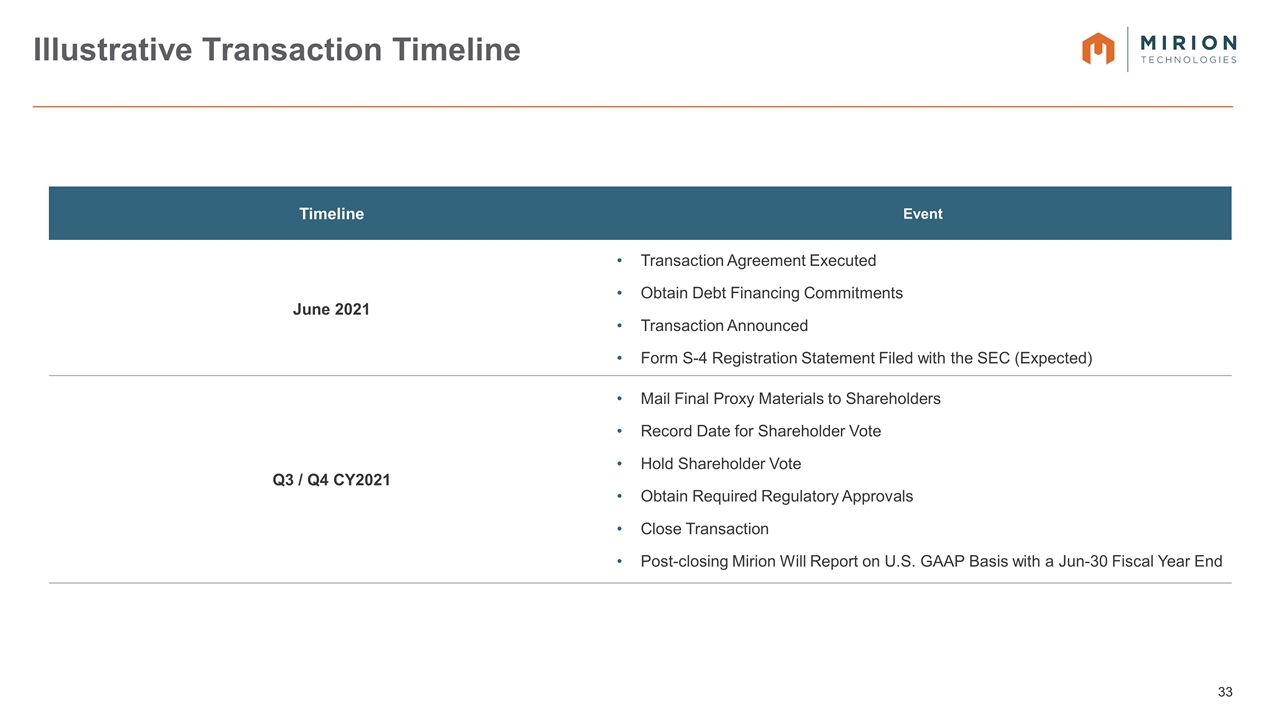
Illustrative Transaction Timeline Timeline Event June 2021 Transaction Agreement Executed Obtain Debt Financing Commitments Transaction Announced Form S-4 Registration Statement Filed with the SEC (Expected) Q3 / Q4 CY2021 Mail Final Proxy Materials to Shareholders Record Date for Shareholder Vote Hold Shareholder Vote Obtain Required Regulatory Approvals Close Transaction Post-closing Mirion Will Report on U.S. GAAP Basis with a Jun-30 Fiscal Year End
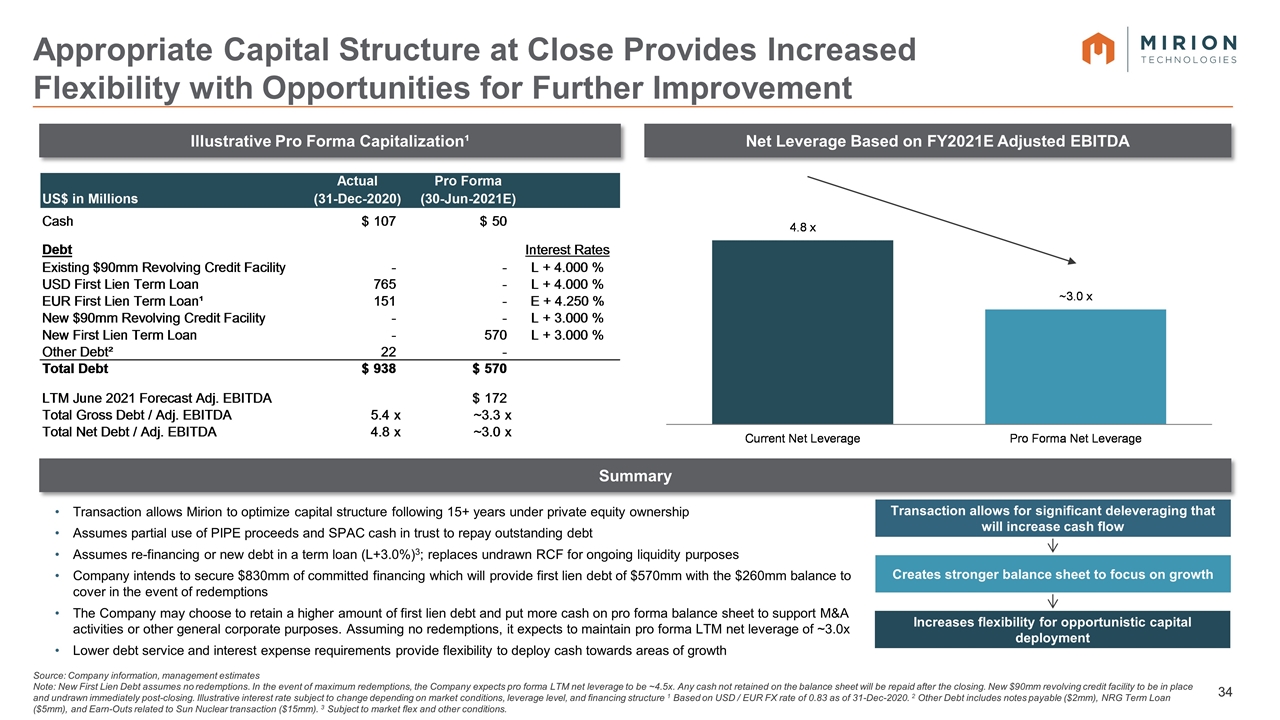
Transaction allows Mirion to optimize capital structure following 15+ years under private equity ownership Assumes partial use of PIPE proceeds and SPAC cash in trust to repay outstanding debt Assumes re-financing or new debt in a term loan (L+3.0%)3; replaces undrawn RCF for ongoing liquidity purposes Company intends to secure $830mm of committed financing which will provide first lien debt of $570mm with the $260mm balance to cover in the event of redemptions The Company may choose to retain a higher amount of first lien debt and put more cash on pro forma balance sheet to support M&A activities or other general corporate purposes. Assuming no redemptions, it expects to maintain pro forma LTM net leverage of ~3.0x Lower debt service and interest expense requirements provide flexibility to deploy cash towards areas of growth Transaction allows for significant deleveraging that will increase cash flow Creates stronger balance sheet to focus on growth Increases flexibility for opportunistic capital deployment Illustrative Pro Forma Capitalization¹ Net Leverage Based on FY2021E Adjusted EBITDA Summary Source: Company information, management estimates Note: New First Lien Debt assumes no redemptions. In the event of maximum redemptions, the Company expects pro forma LTM net leverage to be ~4.5x. Any cash not retained on the balance sheet will be repaid after the closing. New $90mm revolving credit facility to be in place and undrawn immediately post-closing. Illustrative interest rate subject to change depending on market conditions, leverage level, and financing structure 1 Based on USD / EUR FX rate of 0.83 as of 31-Dec-2020. 2 Other Debt includes notes payable ($2mm), NRG Term Loan ($5mm), and Earn-Outs related to Sun Nuclear transaction ($15mm). 3 Subject to market flex and other conditions. Appropriate Capital Structure at Close Provides Increased Flexibility with Opportunities for Further Improvement
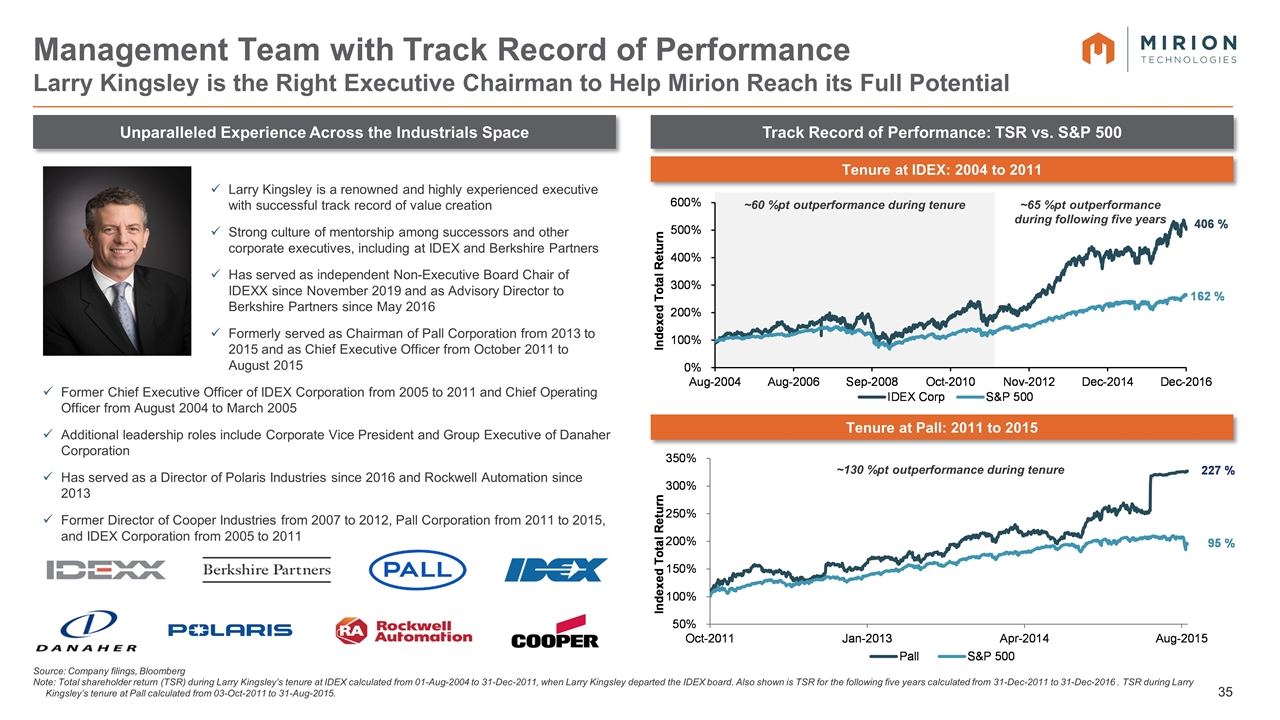
~60 %pt outperformance during tenure Larry Kingsley is a renowned and highly experienced executive with successful track record of value creation Strong culture of mentorship among successors and other corporate executives, including at IDEX and Berkshire Partners Has served as independent Non-Executive Board Chair of IDEXX since November 2019 and as Advisory Director to Berkshire Partners since May 2016 Formerly served as Chairman of Pall Corporation from 2013 to 2015 and as Chief Executive Officer from October 2011 to August 2015 Former Chief Executive Officer of IDEX Corporation from 2005 to 2011 and Chief Operating Officer from August 2004 to March 2005 Additional leadership roles include Corporate Vice President and Group Executive of Danaher Corporation Has served as a Director of Polaris Industries since 2016 and Rockwell Automation since 2013 Former Director of Cooper Industries from 2007 to 2012, Pall Corporation from 2011 to 2015, and IDEX Corporation from 2005 to 2011 Unparalleled Experience Across the Industrials Space Track Record of Performance: TSR vs. S&P 500 Management Team with Track Record of Performance Larry Kingsley is the Right Executive Chairman to Help Mirion Reach its Full Potential Source: Company filings, Bloomberg Note: Total shareholder return (TSR) during Larry Kingsley’s tenure at IDEX calculated from 01-Aug-2004 to 31-Dec-2011, when Larry Kingsley departed the IDEX board. Also shown is TSR for the following five years calculated from 31-Dec-2011 to 31-Dec-2016 . TSR during Larry Kingsley’s tenure at Pall calculated from 03-Oct-2011 to 31-Aug-2015. Tenure at IDEX: 2004 to 2011 Tenure at Pall: 2011 to 2015 ~65 %pt outperformance during following five years ~130 %pt outperformance during tenure
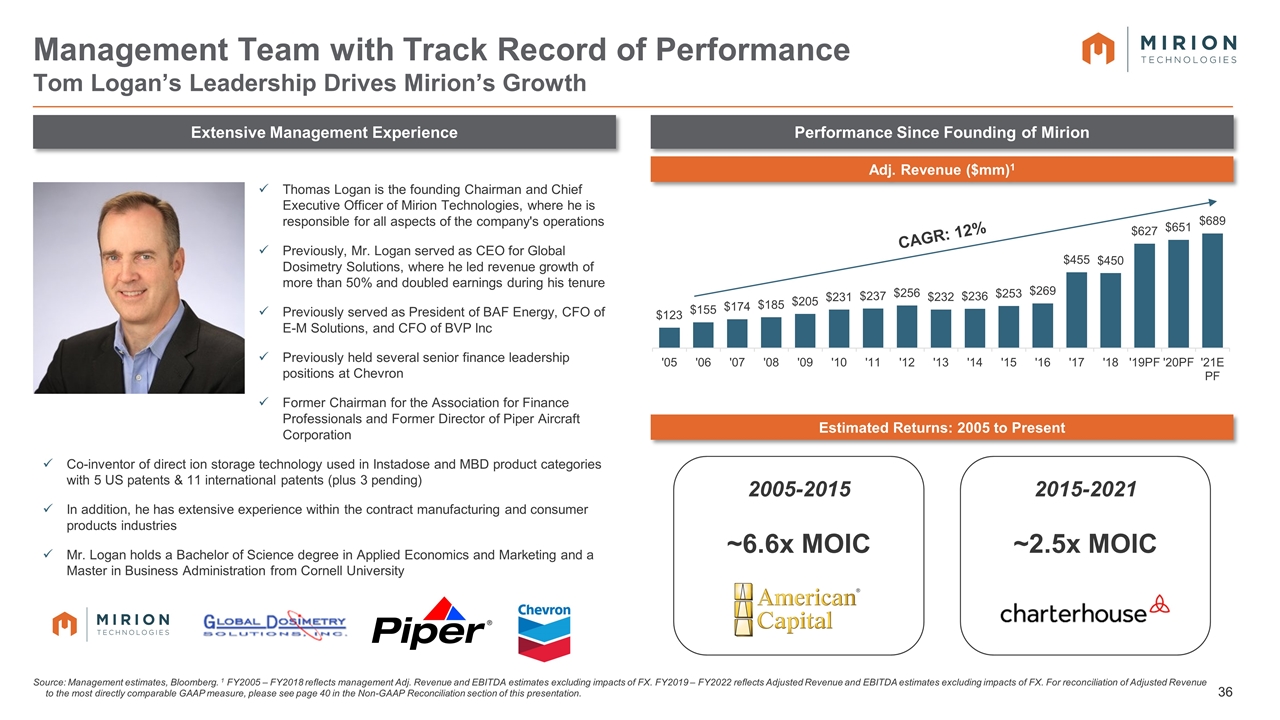
Mirion Founding to Charterhouse Acquisition: +500 %pt Thomas Logan is the founding Chairman and Chief Executive Officer of Mirion Technologies, where he is responsible for all aspects of the company's operations Previously, Mr. Logan served as CEO for Global Dosimetry Solutions, where he led revenue growth of more than 50% and doubled earnings during his tenure Previously served as President of BAF Energy, CFO of E-M Solutions, and CFO of BVP Inc Previously held several senior finance leadership positions at Chevron Former Chairman for the Association for Finance Professionals and Former Director of Piper Aircraft Corporation Co-inventor of direct ion storage technology used in Instadose and MBD product categories with 5 US patents & 11 international patents (plus 3 pending) In addition, he has extensive experience within the contract manufacturing and consumer products industries Mr. Logan holds a Bachelor of Science degree in Applied Economics and Marketing and a Master in Business Administration from Cornell University Extensive Management Experience Performance Since Founding of Mirion Management Team with Track Record of Performance Tom Logan’s Leadership Drives Mirion’s Growth Source: Management estimates, Bloomberg. 1 FY2005 – FY2018 reflects management Adj. Revenue and EBITDA estimates excluding impacts of FX. FY2019 – FY2022 reflects Adjusted Revenue and EBITDA estimates excluding impacts of FX. For reconciliation of Adjusted Revenue to the most directly comparable GAAP measure, please see page 40 in the Non-GAAP Reconciliation section of this presentation. Adj. Revenue ($mm)1 CAGR: 12% Estimated Returns: 2005 to Present 2005-2015 ~6.6x MOIC 2015-2021 ~2.5x MOIC
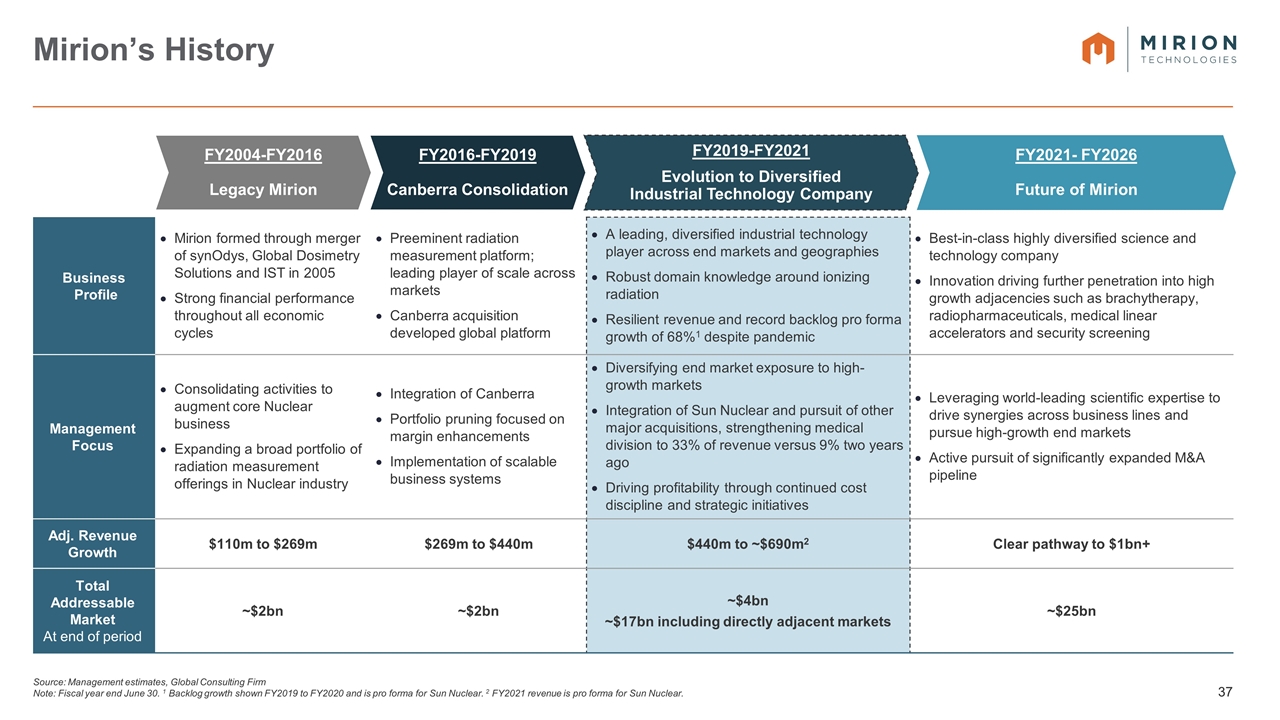
Source: Management estimates, Global Consulting Firm Note: Fiscal year end June 30. 1 Backlog growth shown FY2019 to FY2020 and is pro forma for Sun Nuclear. 2 FY2021 revenue is pro forma for Sun Nuclear. Mirion’s History Business Profile Mirion formed through merger of synOdys, Global Dosimetry Solutions and IST in 2005 Strong financial performance throughout all economic cycles Preeminent radiation measurement platform; leading player of scale across markets Canberra acquisition developed global platform A leading, diversified industrial technology player across end markets and geographies Robust domain knowledge around ionizing radiation Resilient revenue and record backlog pro forma growth of 68%1 despite pandemic Best-in-class highly diversified science and technology company Innovation driving further penetration into high growth adjacencies such as brachytherapy, radiopharmaceuticals, medical linear accelerators and security screening Management Focus Consolidating activities to augment core Nuclear business Expanding a broad portfolio of radiation measurement offerings in Nuclear industry Integration of Canberra Portfolio pruning focused on margin enhancements Implementation of scalable business systems Diversifying end market exposure to high-growth markets Integration of Sun Nuclear and pursuit of other major acquisitions, strengthening medical division to 33% of revenue versus 9% two years ago Driving profitability through continued cost discipline and strategic initiatives Leveraging world-leading scientific expertise to drive synergies across business lines and pursue high-growth end markets Active pursuit of significantly expanded M&A pipeline Adj. Revenue Growth $110m to $269m $269m to $440m $440m to ~$690m2 Clear pathway to $1bn+ Total Addressable Market At end of period ~$2bn ~$2bn ~$4bn ~$17bn including directly adjacent markets ~$25bn FY2004-FY2016 Legacy Mirion FY2019-FY2021 Evolution to Diversified Industrial Technology Company FY2016-FY2019 Canberra Consolidation FY2021- FY2026 Future of Mirion
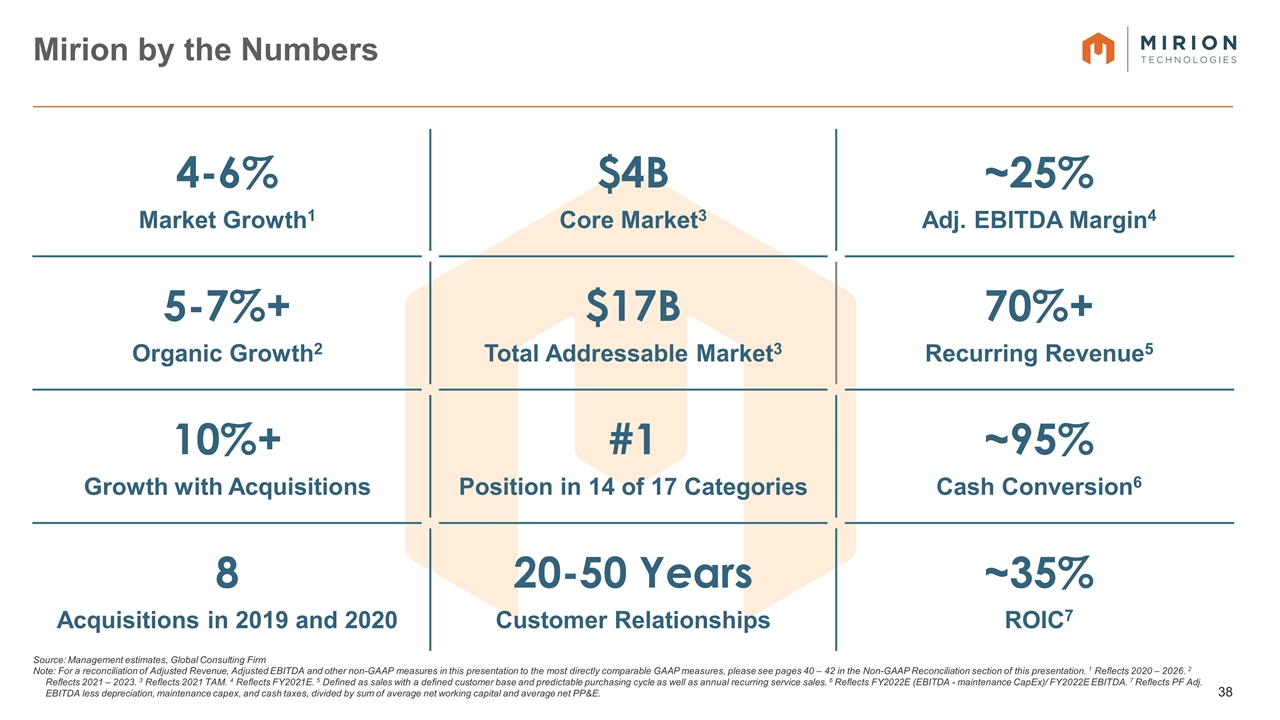
4-6% Market Growth1 $4B Core Market3 ~25% Adj. EBITDA Margin4 5-7%+ Organic Growth2 $17B Total Addressable Market3 70%+ Recurring Revenue5 10%+ Growth with Acquisitions #1 Position in 14 of 17 Categories ~95% Cash Conversion6 8 Acquisitions in 2019 and 2020 20-50 Years Customer Relationships ~35% ROIC7 Source: Management estimates, Global Consulting Firm Note: For a reconciliation of Adjusted Revenue, Adjusted EBITDA and other non-GAAP measures in this presentation to the most directly comparable GAAP measures, please see pages 40 – 42 in the Non-GAAP Reconciliation section of this presentation. 1 Reflects 2020 – 2026. 2 Reflects 2021 – 2023. 3 Reflects 2021 TAM. 4 Reflects FY2021E. 5 Defined as sales with a defined customer base and predictable purchasing cycle as well as annual recurring service sales. 6 Reflects FY2022E (EBITDA - maintenance CapEx)/ FY2022E EBITDA. 7 Reflects PF Adj. EBITDA less depreciation, maintenance capex, and cash taxes, divided by sum of average net working capital and average net PP&E. Mirion by the Numbers

Non-GAAP Reconciliation
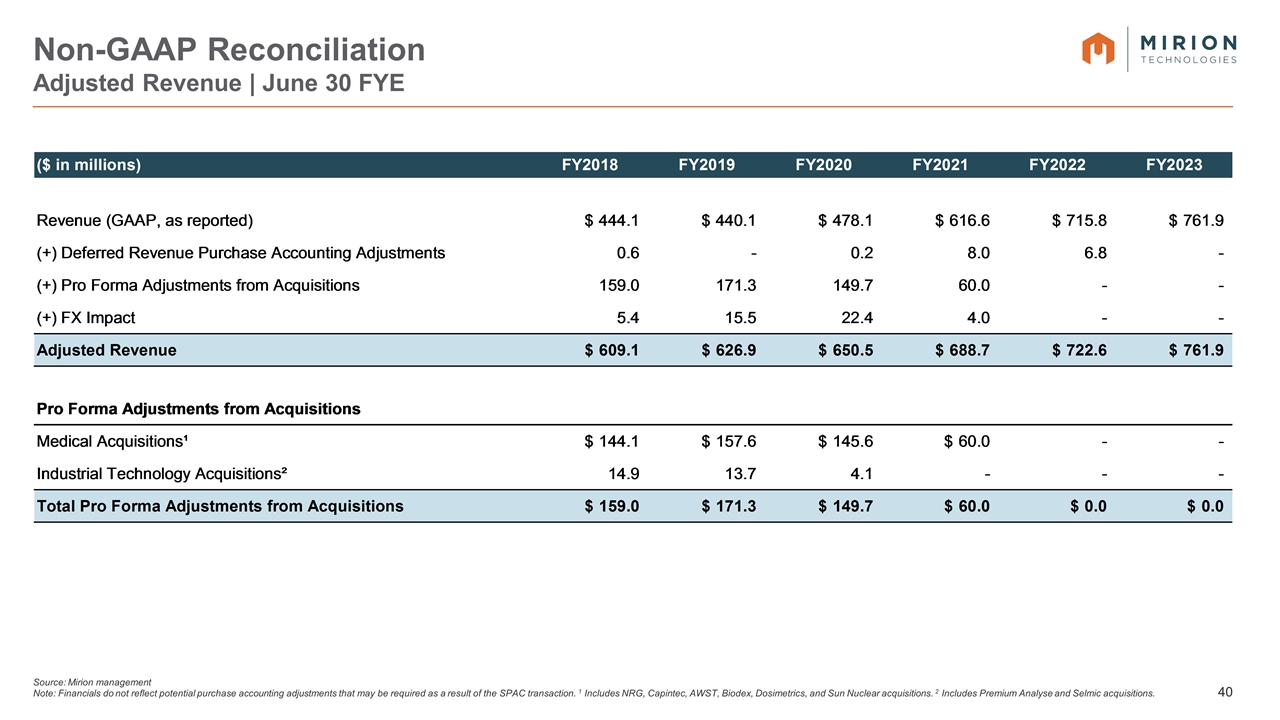
Source: Mirion management Note: Financials do not reflect potential purchase accounting adjustments that may be required as a result of the SPAC transaction. 1 Includes NRG, Capintec, AWST, Biodex, Dosimetrics, and Sun Nuclear acquisitions. 2 Includes Premium Analyse and Selmic acquisitions. Non-GAAP Reconciliation Adjusted Revenue | June 30 FYE
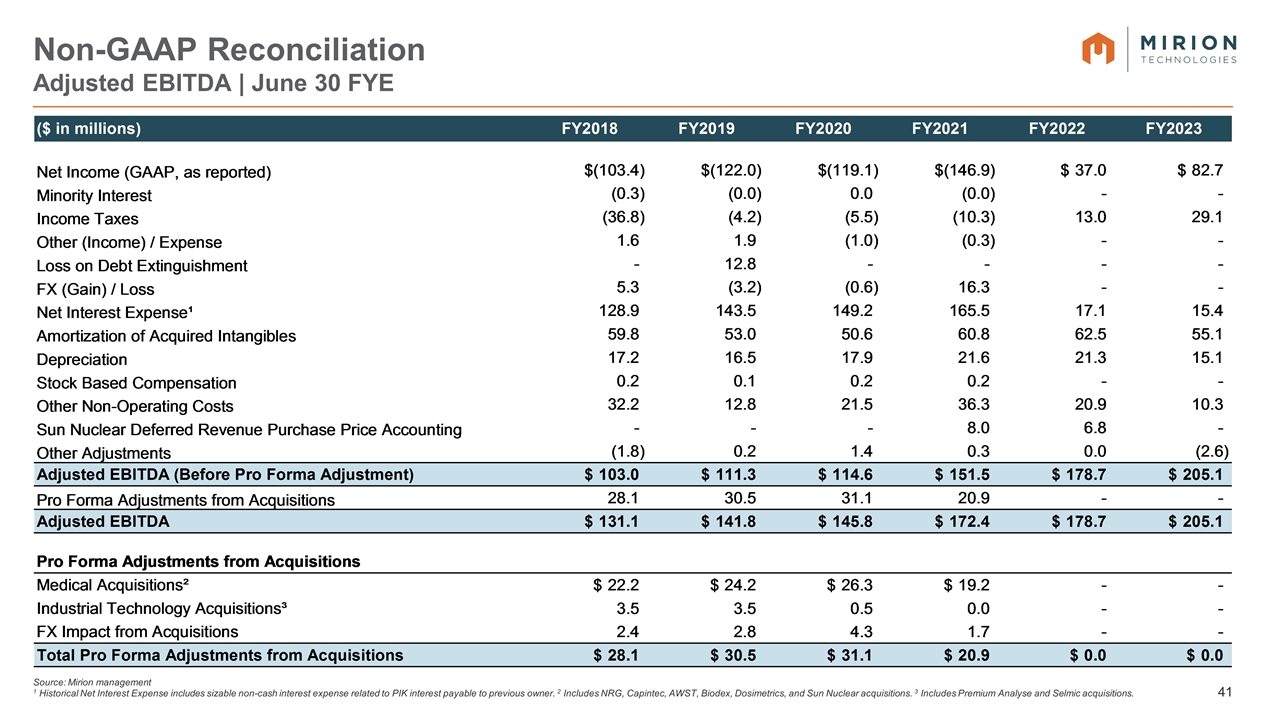
Source: Mirion management 1 Historical Net Interest Expense includes sizable non-cash interest expense related to PIK interest payable to previous owner. 2 Includes NRG, Capintec, AWST, Biodex, Dosimetrics, and Sun Nuclear acquisitions. 3 Includes Premium Analyse and Selmic acquisitions. Non-GAAP Reconciliation Adjusted EBITDA | June 30 FYE
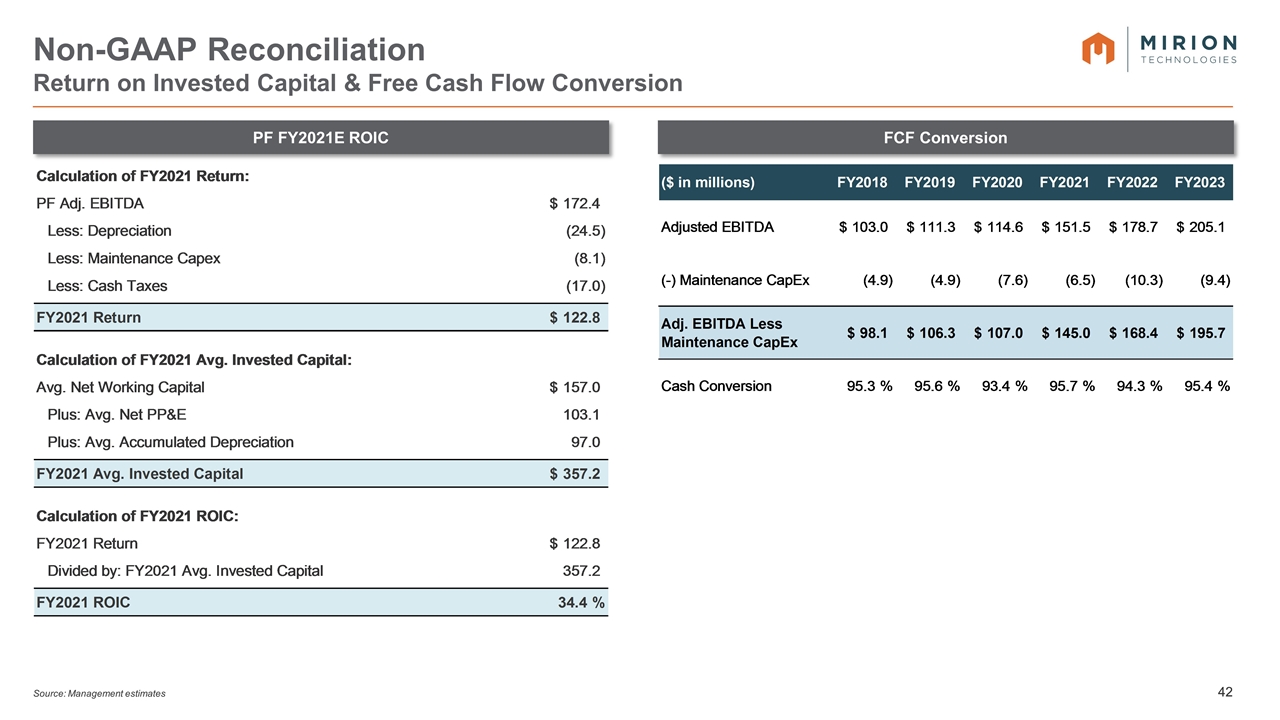
Source: Management estimates Non-GAAP Reconciliation Return on Invested Capital & Free Cash Flow Conversion PF FY2021E ROIC FCF Conversion

Nuclear Market Overview
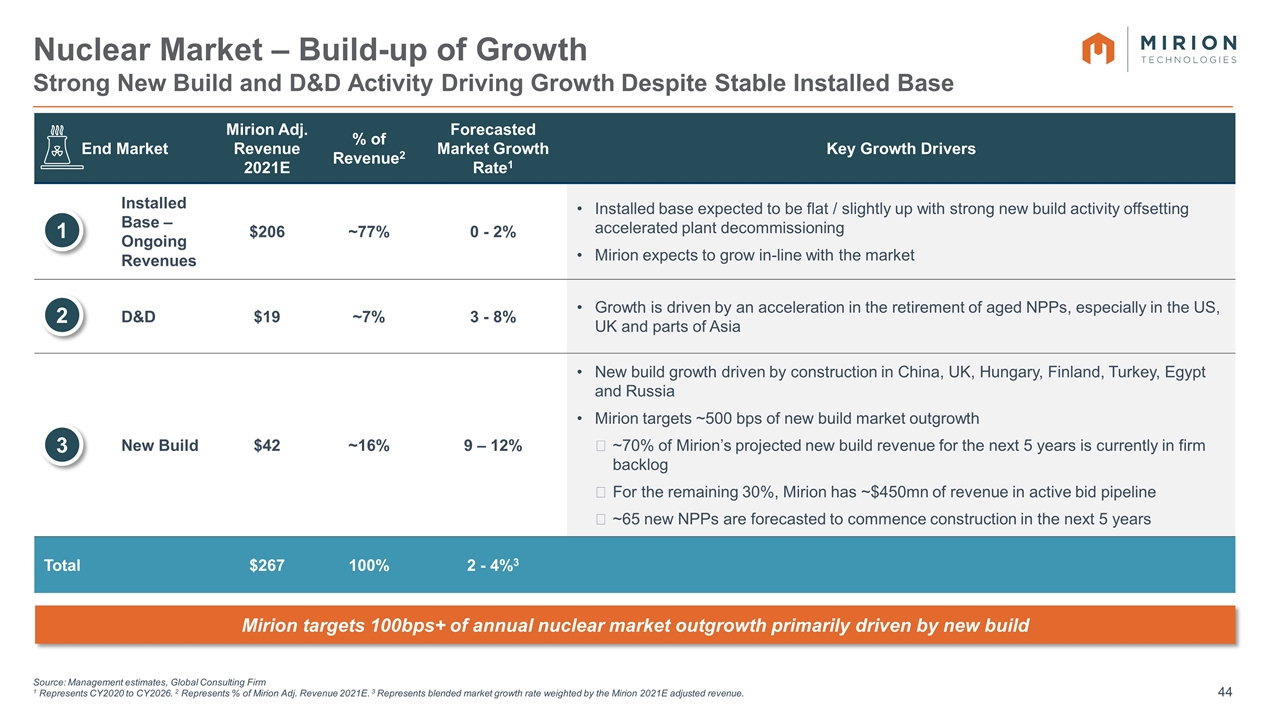
Source: Management estimates, Global Consulting Firm 1 Represents CY2020 to CY2026. 2 Represents % of Mirion Adj. Revenue 2021E. 3 Represents blended market growth rate weighted by the Mirion 2021E adjusted revenue. Nuclear Market – Build-up of Growth Strong New Build and D&D Activity Driving Growth Despite Stable Installed Base End Market Mirion Adj. Revenue 2021E % of Revenue2 Forecasted Market Growth Rate1 Key Growth Drivers Installed Base – Ongoing Revenues $206 ~77% 0 - 2% Installed base expected to be flat / slightly up with strong new build activity offsetting accelerated plant decommissioning Mirion expects to grow in-line with the market D&D $19 ~7% 3 - 8% Growth is driven by an acceleration in the retirement of aged NPPs, especially in the US, UK and parts of Asia New Build $42 ~16% 9 – 12% New build growth driven by construction in China, UK, Hungary, Finland, Turkey, Egypt and Russia Mirion targets ~500 bps of new build market outgrowth ~70% of Mirion’s projected new build revenue for the next 5 years is currently in firm backlog For the remaining 30%, Mirion has ~$450mn of revenue in active bid pipeline ~65 new NPPs are forecasted to commence construction in the next 5 years Total $267 100% 2 - 4%3 1 2 3 Mirion targets 100bps+ of annual nuclear market outgrowth primarily driven by new build
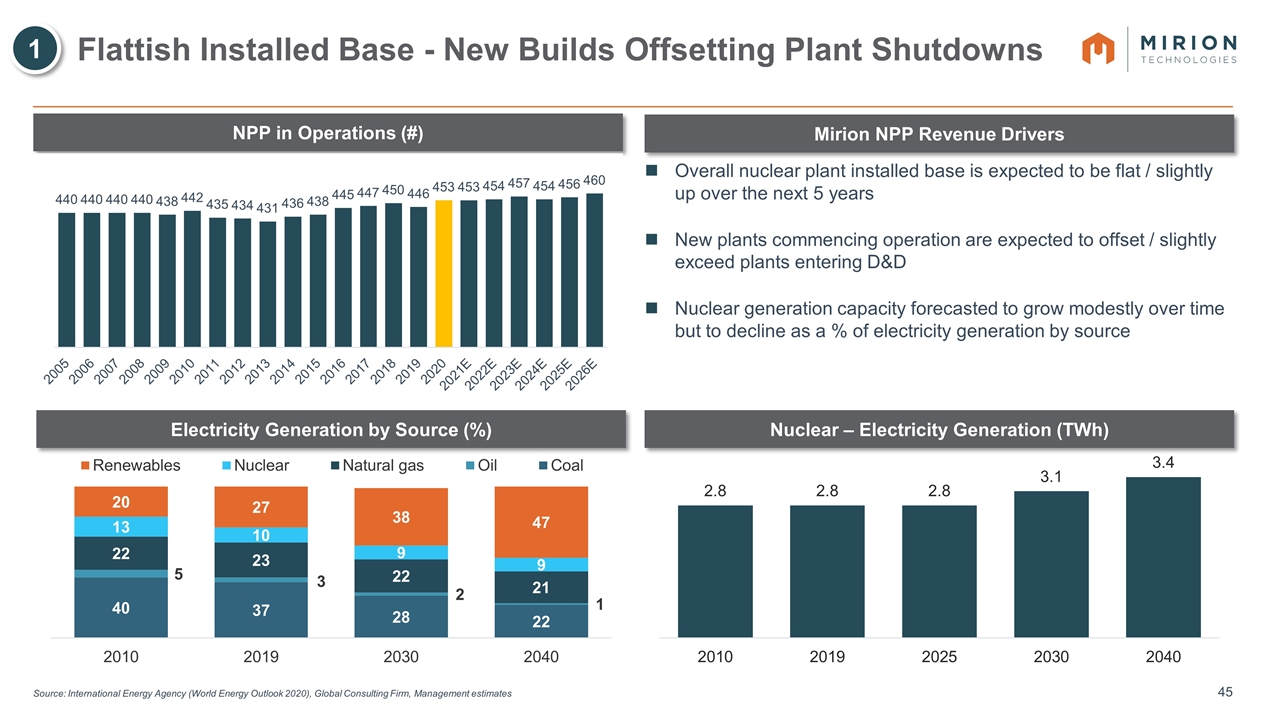
Source: International Energy Agency (World Energy Outlook 2020), Global Consulting Firm, Management estimates Flattish Installed Base - New Builds Offsetting Plant Shutdowns Electricity Generation by Source (%) Nuclear – Electricity Generation (TWh) NPP in Operations (#) Mirion NPP Revenue Drivers Overall nuclear plant installed base is expected to be flat / slightly up over the next 5 years New plants commencing operation are expected to offset / slightly exceed plants entering D&D Nuclear generation capacity forecasted to grow modestly over time but to decline as a % of electricity generation by source 1
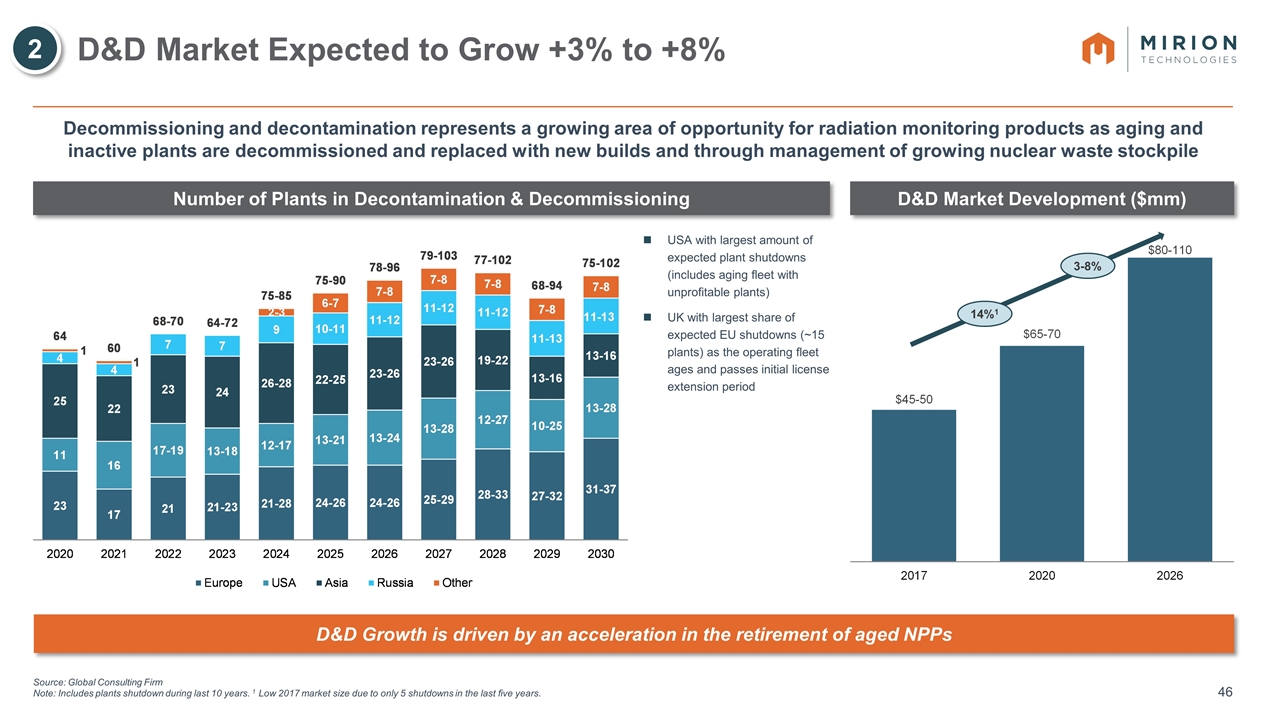
Source: Global Consulting Firm Note: Includes plants shutdown during last 10 years. 1 Low 2017 market size due to only 5 shutdowns in the last five years. D&D Market Expected to Grow +3% to +8% 2 Number of Plants in Decontamination & Decommissioning D&D Market Development ($mm) USA with largest amount of expected plant shutdowns (includes aging fleet with unprofitable plants) UK with largest share of expected EU shutdowns (~15 plants) as the operating fleet ages and passes initial license extension period Decommissioning and decontamination represents a growing area of opportunity for radiation monitoring products as aging and inactive plants are decommissioned and replaced with new builds and through management of growing nuclear waste stockpile 14%1 3-8% D&D Growth is driven by an acceleration in the retirement of aged NPPs
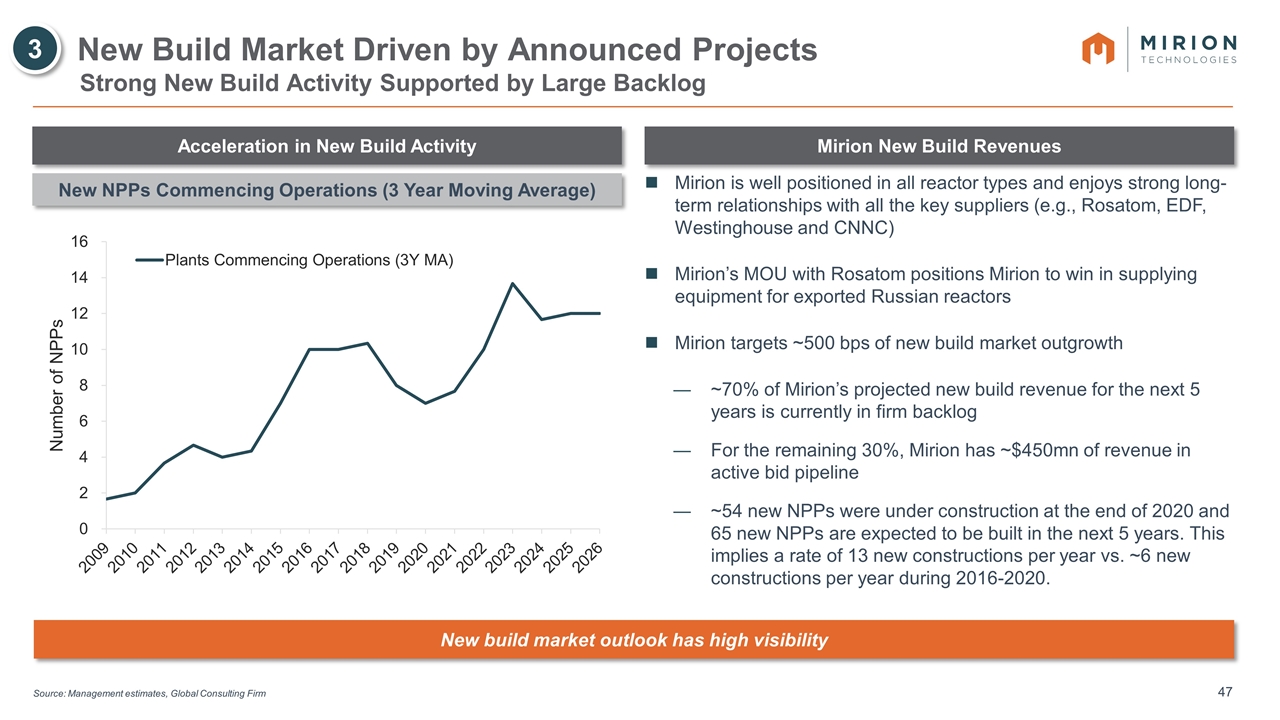
Source: Management estimates, Global Consulting Firm New Build Market Driven by Announced Projects 3 Strong New Build Activity Supported by Large Backlog Acceleration in New Build Activity Mirion New Build Revenues Mirion is well positioned in all reactor types and enjoys strong long-term relationships with all the key suppliers (e.g., Rosatom, EDF, Westinghouse and CNNC) Mirion’s MOU with Rosatom positions Mirion to win in supplying equipment for exported Russian reactors Mirion targets ~500 bps of new build market outgrowth ~70% of Mirion’s projected new build revenue for the next 5 years is currently in firm backlog For the remaining 30%, Mirion has ~$450mn of revenue in active bid pipeline ~54 new NPPs were under construction at the end of 2020 and 65 new NPPs are expected to be built in the next 5 years. This implies a rate of 13 new constructions per year vs. ~6 new constructions per year during 2016-2020. New NPPs Commencing Operations (3 Year Moving Average) New build market outlook has high visibility
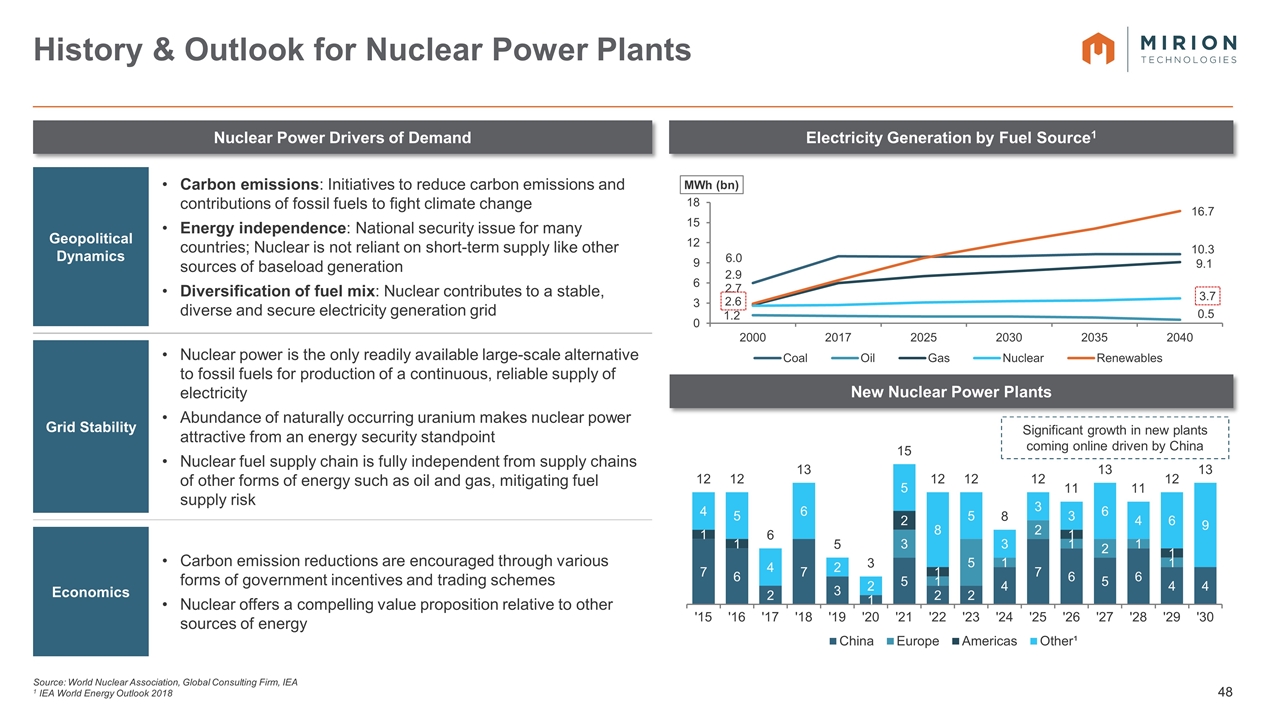
Source: World Nuclear Association, Global Consulting Firm, IEA 1 IEA World Energy Outlook 2018 History & Outlook for Nuclear Power Plants Geopolitical Dynamics Carbon emissions: Initiatives to reduce carbon emissions and contributions of fossil fuels to fight climate change Energy independence: National security issue for many countries; Nuclear is not reliant on short-term supply like other sources of baseload generation Diversification of fuel mix: Nuclear contributes to a stable, diverse and secure electricity generation grid Grid Stability Nuclear power is the only readily available large-scale alternative to fossil fuels for production of a continuous, reliable supply of electricity Abundance of naturally occurring uranium makes nuclear power attractive from an energy security standpoint Nuclear fuel supply chain is fully independent from supply chains of other forms of energy such as oil and gas, mitigating fuel supply risk Economics Carbon emission reductions are encouraged through various forms of government incentives and trading schemes Nuclear offers a compelling value proposition relative to other sources of energy Nuclear Power Drivers of Demand Electricity Generation by Fuel Source1 New Nuclear Power Plants
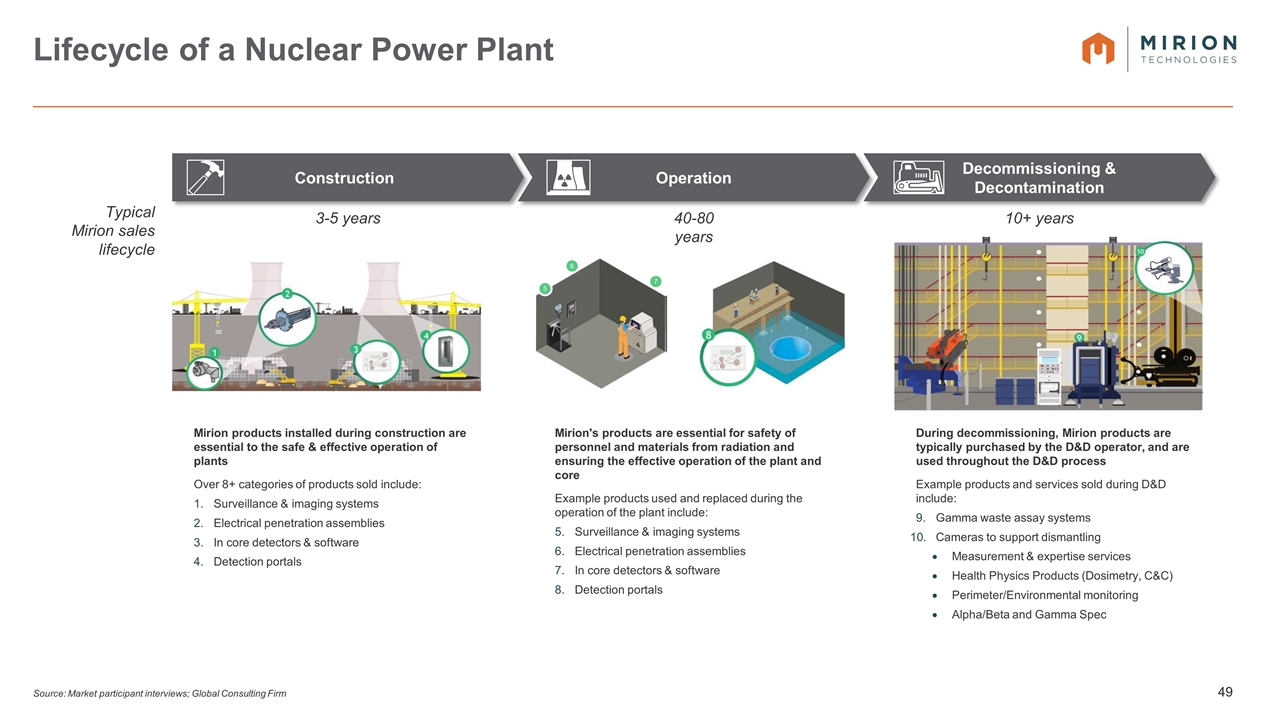
Source: Market participant interviews; Global Consulting Firm Lifecycle of a Nuclear Power Plant 5 6 7 Mirion products installed during construction are essential to the safe & effective operation of plants Over 8+ categories of products sold include: Surveillance & imaging systems Electrical penetration assemblies In core detectors & software Detection portals Mirion's products are essential for safety of personnel and materials from radiation and ensuring the effective operation of the plant and core Example products used and replaced during the operation of the plant include: Surveillance & imaging systems Electrical penetration assemblies In core detectors & software Detection portals During decommissioning, Mirion products are typically purchased by the D&D operator, and are used throughout the D&D process Example products and services sold during D&D include: Gamma waste assay systems Cameras to support dismantling Measurement & expertise services Health Physics Products (Dosimetry, C&C) Perimeter/Environmental monitoring Alpha/Beta and Gamma Spec Typical Mirion sales lifecycle 3-5 years 40-80 years 10+ years Operation Decommissioning & Decontamination Construction
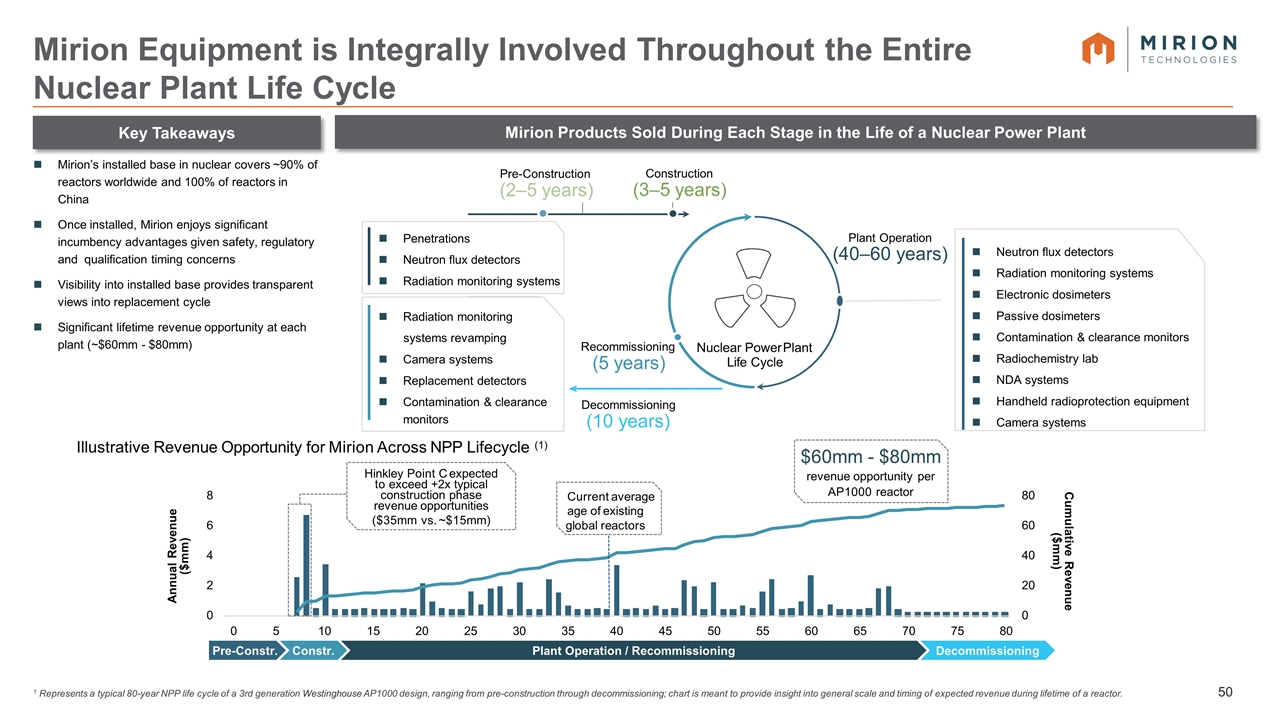
1 Represents a typical 80-year NPP life cycle of a 3rd generation Westinghouse AP1000 design, ranging from pre-construction through decommissioning; chart is meant to provide insight into general scale and timing of expected revenue during lifetime of a reactor. Mirion Equipment is Integrally Involved Throughout the Entire Nuclear Plant Life Cycle Key Takeaways Mirion Products Sold During Each Stage in the Life of a Nuclear Power Plant Mirion’s installed base in nuclear covers ~90% of reactors worldwide and 100% of reactors in China Once installed, Mirion enjoys significant incumbency advantages given safety, regulatory and qualification timing concerns Visibility into installed base provides transparent views into replacement cycle Significant lifetime revenue opportunity at each plant (~$60mm - $80mm) Nuclear Power Plant Life Cycle Recommissioning (5 years) Plant Operation (40–60 years) Decommissioning (10 years) Pre-Construction (2–5 years) Construction (3–5 years) Radiation monitoring systems revamping Camera systems Replacement detectors Contamination & clearance monitors Penetrations Neutron flux detectors Radiation monitoring systems Neutron flux detectors Radiation monitoring systems Electronic dosimeters Passive dosimeters Contamination & clearance monitors Radiochemistry lab NDA systems Handheld radioprotection equipment Camera systems Illustrative Revenue Opportunity for Mirion Across NPP Lifecycle (1) $60mm - $80mm revenue opportunity per AP1000 reactor 0 20 40 60 80 0 2 4 6 8 Annual Revenue ($mm) Cumulative Revenue ($mm) Current average age of existing global reactors Hinkley Point C expected to exceed +2x typical construction phase revenue opportunities ($35mm vs. ~$15mm) 05 80 10 15 20 25 30 35 40 45 50 55 60 65 70 75 Pre-Constr. Constr. Plant Operation / Recommissioning Decommissioning
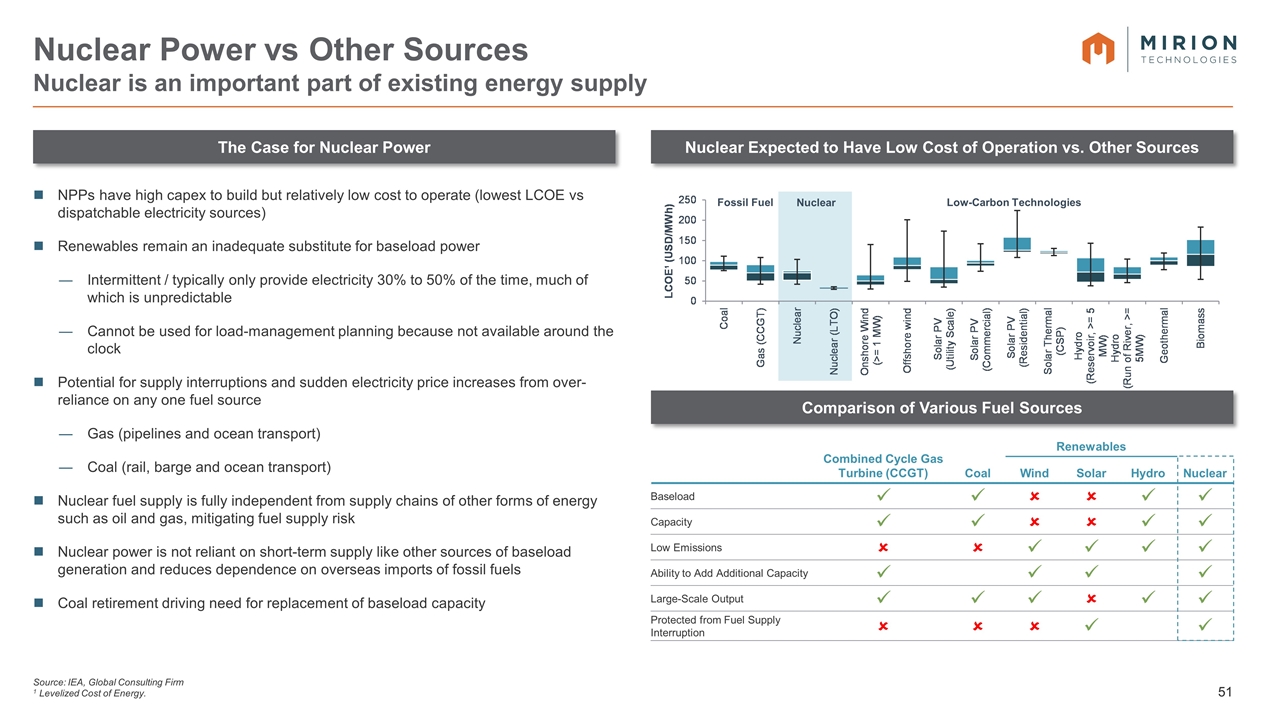
The Case for Nuclear Power Nuclear Expected to Have Low Cost of Operation vs. Other Sources NPPs have high capex to build but relatively low cost to operate (lowest LCOE vs dispatchable electricity sources) Renewables remain an inadequate substitute for baseload power Intermittent / typically only provide electricity 30% to 50% of the time, much of which is unpredictable Cannot be used for load-management planning because not available around the clock Potential for supply interruptions and sudden electricity price increases from over-reliance on any one fuel source Gas (pipelines and ocean transport) Coal (rail, barge and ocean transport) Nuclear fuel supply is fully independent from supply chains of other forms of energy such as oil and gas, mitigating fuel supply risk Nuclear power is not reliant on short-term supply like other sources of baseload generation and reduces dependence on overseas imports of fossil fuels Coal retirement driving need for replacement of baseload capacity Nuclear Power vs Other Sources Nuclear is an important part of existing energy supply Source: IEA, Global Consulting Firm 1 Levelized Cost of Energy. Comparison of Various Fuel Sources Combined Cycle Gas Turbine (CCGT) Renewables Coal Wind Solar Hydro Nuclear Baseload ü ü û û ü ü Capacity ü ü û û ü ü Low Emissions û û ü ü ü ü Ability to Add Additional Capacity ü ü ü ü Large-Scale Output ü ü ü û ü ü Protected from Fuel Supply Interruption û û û ü ü Low-Carbon Technologies Fossil Fuel Nuclear
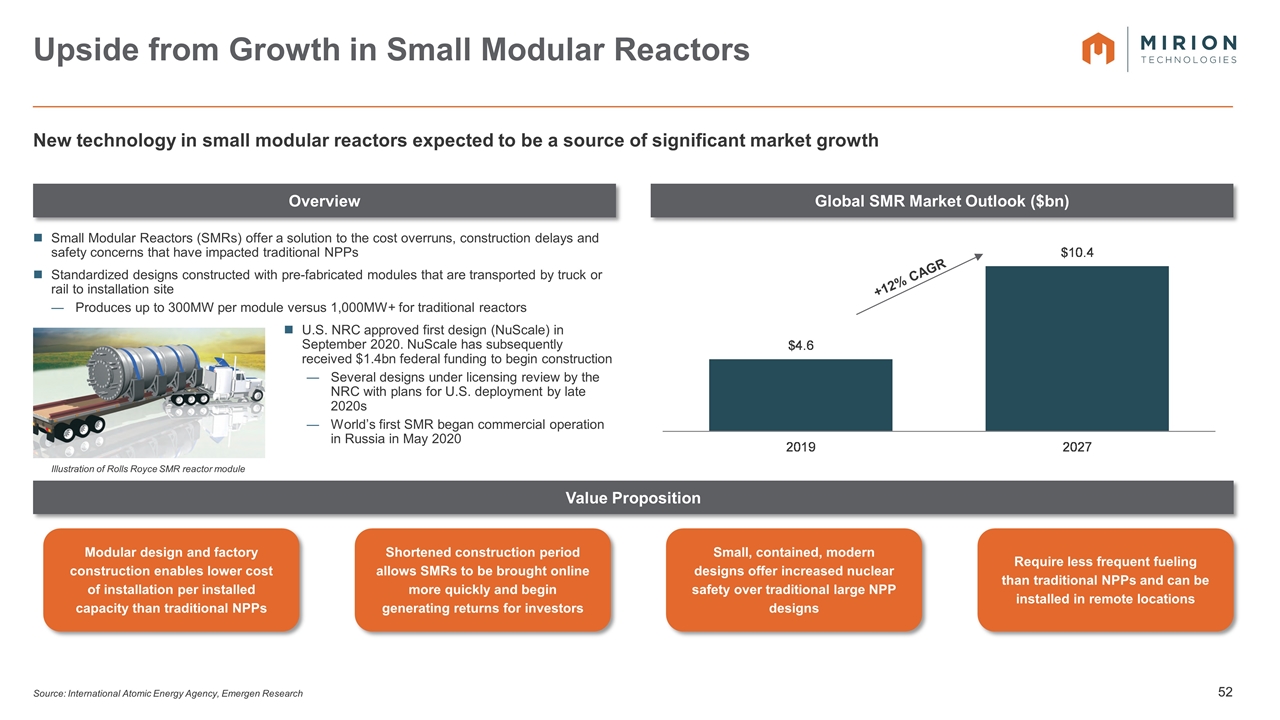
Small Modular Reactors (SMRs) offer a solution to the cost overruns, construction delays and safety concerns that have impacted traditional NPPs Standardized designs constructed with pre-fabricated modules that are transported by truck or rail to installation site Produces up to 300MW per module versus 1,000MW+ for traditional reactors U.S. NRC approved first design (NuScale) in September 2020. NuScale has subsequently received $1.4bn federal funding to begin construction Several designs under licensing review by the NRC with plans for U.S. deployment by late 2020s World’s first SMR began commercial operation in Russia in May 2020 Overview Global SMR Market Outlook ($bn) Value Proposition Upside from Growth in Small Modular Reactors Source: International Atomic Energy Agency, Emergen Research New technology in small modular reactors expected to be a source of significant market growth Illustration of Rolls Royce SMR reactor module Modular design and factory construction enables lower cost of installation per installed capacity than traditional NPPs Shortened construction period allows SMRs to be brought online more quickly and begin generating returns for investors Small, contained, modern designs offer increased nuclear safety over traditional large NPP designs Require less frequent fueling than traditional NPPs and can be installed in remote locations

Medical Market Overview
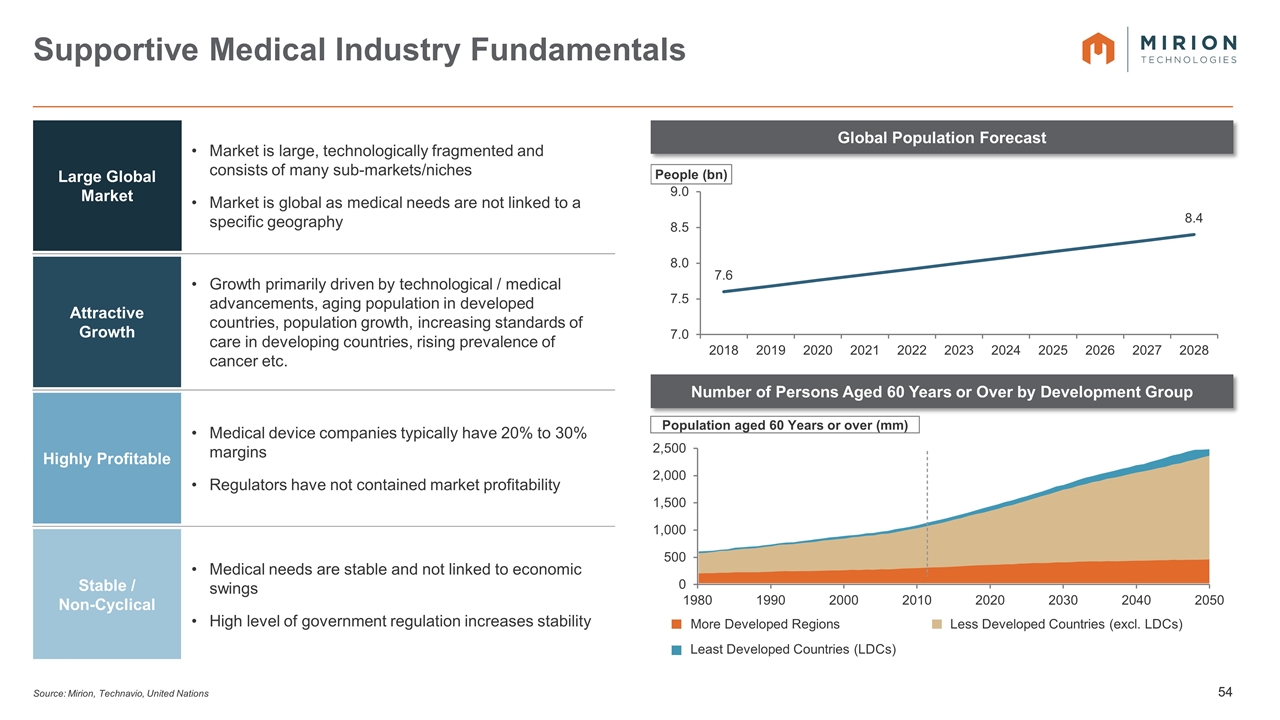
Source: Mirion, Technavio, United Nations Supportive Medical Industry Fundamentals Global Population Forecast Number of Persons Aged 60 Years or Over by Development Group Large Global Market Market is large, technologically fragmented and consists of many sub-markets/niches Market is global as medical needs are not linked to a specific geography Attractive Growth Growth primarily driven by technological / medical advancements, aging population in developed countries, population growth, increasing standards of care in developing countries, rising prevalence of cancer etc. Highly Profitable Medical device companies typically have 20% to 30% margins Regulators have not contained market profitability Stable / Non-Cyclical Medical needs are stable and not linked to economic swings High level of government regulation increases stability Population aged 60 Years or over (mm) More Developed Regions Less Developed Countries (excl. LDCs) Least Developed Countries (LDCs)
Growing awareness about the benefits of RT for cancer treatment In U.S., 3.05M patients received radiation therapy in 2016, which is expected to increase by ~37% reaching 4.17M by 2040 Increased cancer patient population Global number of new cancer cases is expected to rise by about 70% over the next two decades Technological advancements in the field of radiotherapy Constant technological advancements in field have led to growth in the radiotherapy market in the past years and will continue to do so Aging population Total population aged 60 years or above reached over 962 million in 2017, and is expected to increase to 2.1 billion by 2050, driving the incidence of various chronic diseases, including cancer Overview Market by Geography Radiation Therapy Market Overview Source: Global Consulting Firm Growing awareness about the benefits of Radiation Therapy for cancer treatment and increased cancer patient population are the key drivers of the radiation therapy market Radiation therapy market is expected to be valued at $7.1 billion in 2020 and is projected to grow at a 5.7% CAGR during the period of 2020 to 2025 The emerging markets, growing government and private investments to meet the increasing demand for cancer treatment, and the improving reimbursement scenario are expected to present a wide range of growth opportunities for market players The market for RT QA software is growing rapidly, and is estimated at ~$50M in 2019 and ~20% CAGR '19-'24, driven by continued software adoption and uptake in software models Overall, healthy mid-to-high single digits growth expected in core QA market Software with very positive outlook; adoption increasing globally, driven by enhanced offering and clinics' growing appetite for software solutions Hardware expected to grow low single digits; some headwinds in Patient QA from software substitution, while Machine QA largely shielded China expected to experience the fastest growth fueled by population, use of radiation therapy and software growth China lagging in adoption of software indicating growth opportunity Low density of LINACs per population gives overall whitespace for market growth Largest market with fragmented market of small, more cost–conscious private clinics Spending driven by LINAC replacement cycles mainly with continued software adoption Dominated by large hospitals organized in socialized healthcare Spending set by government budgets giving more leeway to adopt software earlier Overall low penetration of radiation therapy and software adoption across emerging markets APAC / RoW Market Drivers Level of importance − + High Low Key Market Themes in Software RT QA
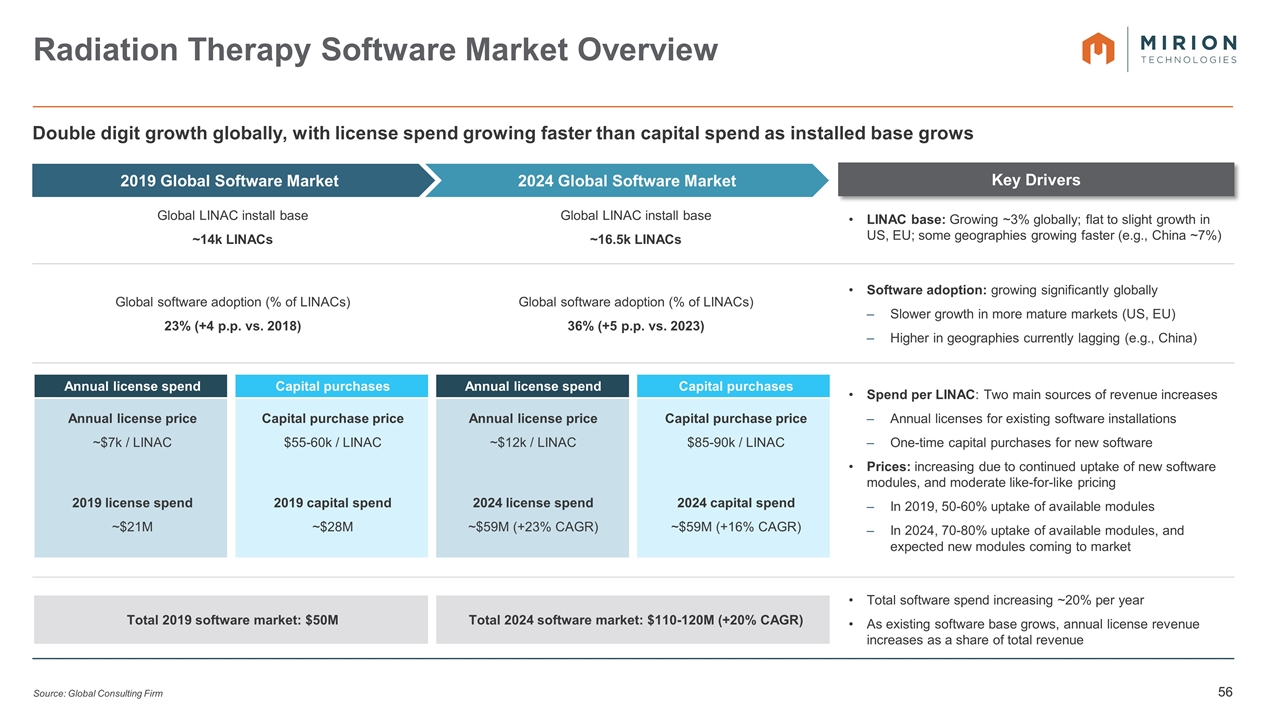
Key Drivers 2019 Global SW Market Size Estimate 2024 Global SW Market Size Estimate Global LINAC install base ~14k LINACs Global LINAC install base ~16.5k LINACs LINAC base: Growing ~3% globally; flat to slight growth in US, EU; some geographies growing faster (e.g., China ~7%) Global software adoption (% of LINACs) 23% (+4 p.p. vs. 2018) Global software adoption (% of LINACs) 36% (+5 p.p. vs. 2023) Software adoption: growing significantly globally Slower growth in more mature markets (US, EU) Higher in geographies currently lagging (e.g., China) Annual license spend Capital purchases Annual license spend Capital purchases Spend per LINAC: Two main sources of revenue increases Annual licenses for existing software installations One-time capital purchases for new software Prices: increasing due to continued uptake of new software modules, and moderate like-for-like pricing In 2019, 50-60% uptake of available modules In 2024, 70-80% uptake of available modules, and expected new modules coming to market Annual license price ~$7k / LINAC Capital purchase price $55-60k / LINAC Annual license price ~$12k / LINAC Capital purchase price $85-90k / LINAC 2019 license spend ~$21M 2019 capital spend ~$28M 2024 license spend ~$59M (+23% CAGR) 2024 capital spend ~$59M (+16% CAGR) Total 2019 software market: $50M Total 2024 software market: $110-120M (+20% CAGR) Total software spend increasing ~20% per year As existing software base grows, annual license revenue increases as a share of total revenue Radiation Therapy Software Market Overview Source: Global Consulting Firm Double digit growth globally, with license spend growing faster than capital spend as installed base grows 2019 Global Software Market 2024 Global Software Market
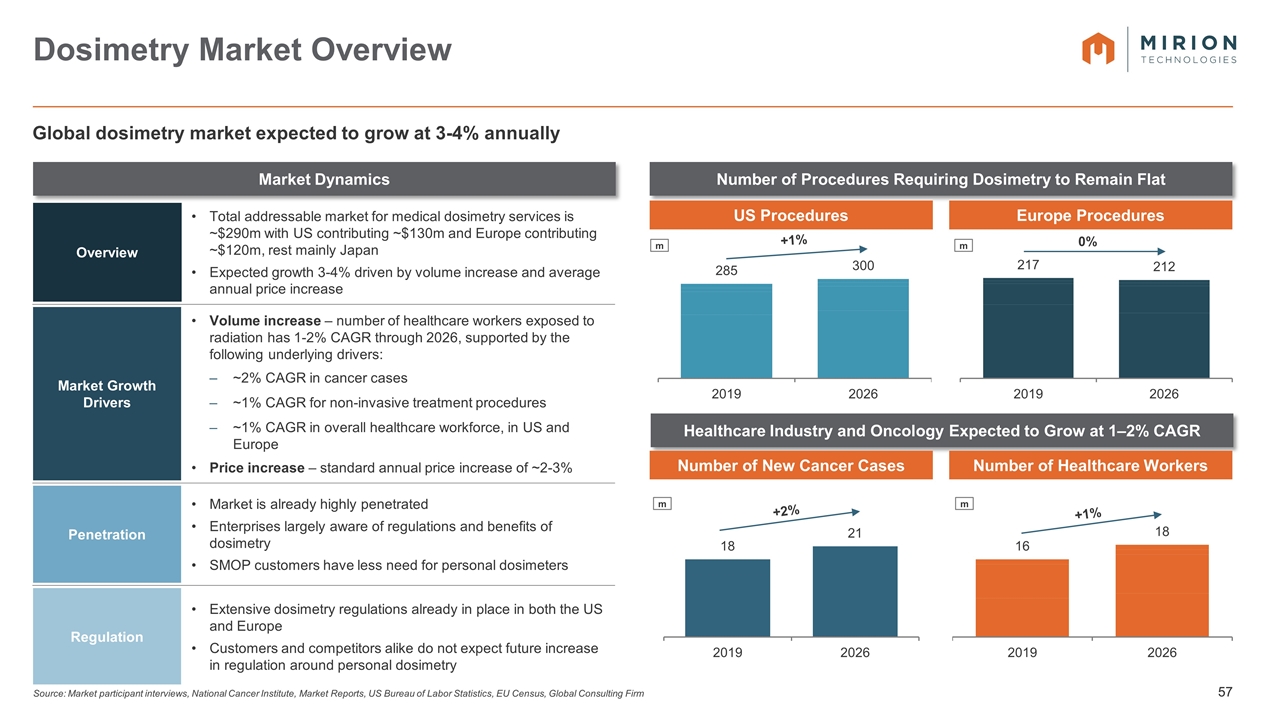
Source: Market participant interviews, National Cancer Institute, Market Reports, US Bureau of Labor Statistics, EU Census, Global Consulting Firm Dosimetry Market Overview Market Dynamics Number of Procedures Requiring Dosimetry to Remain Flat Healthcare Industry and Oncology Expected to Grow at 1–2% CAGR Overview Total addressable market for medical dosimetry services is ~$290m with US contributing ~$130m and Europe contributing ~$120m, rest mainly Japan Expected growth 3-4% driven by volume increase and average annual price increase Market Growth Drivers Volume increase – number of healthcare workers exposed to radiation has 1-2% CAGR through 2026, supported by the following underlying drivers: ~2% CAGR in cancer cases ~1% CAGR for non-invasive treatment procedures ~1% CAGR in overall healthcare workforce, in US and Europe Price increase – standard annual price increase of ~2-3% Penetration Market is already highly penetrated Enterprises largely aware of regulations and benefits of dosimetry SMOP customers have less need for personal dosimeters Regulation Extensive dosimetry regulations already in place in both the US and Europe Customers and competitors alike do not expect future increase in regulation around personal dosimetry Global dosimetry market expected to grow at 3-4% annually US Procedures Europe Procedures Number of New Cancer Cases Number of Healthcare Workers m m +1% 0% m m +2% +1%
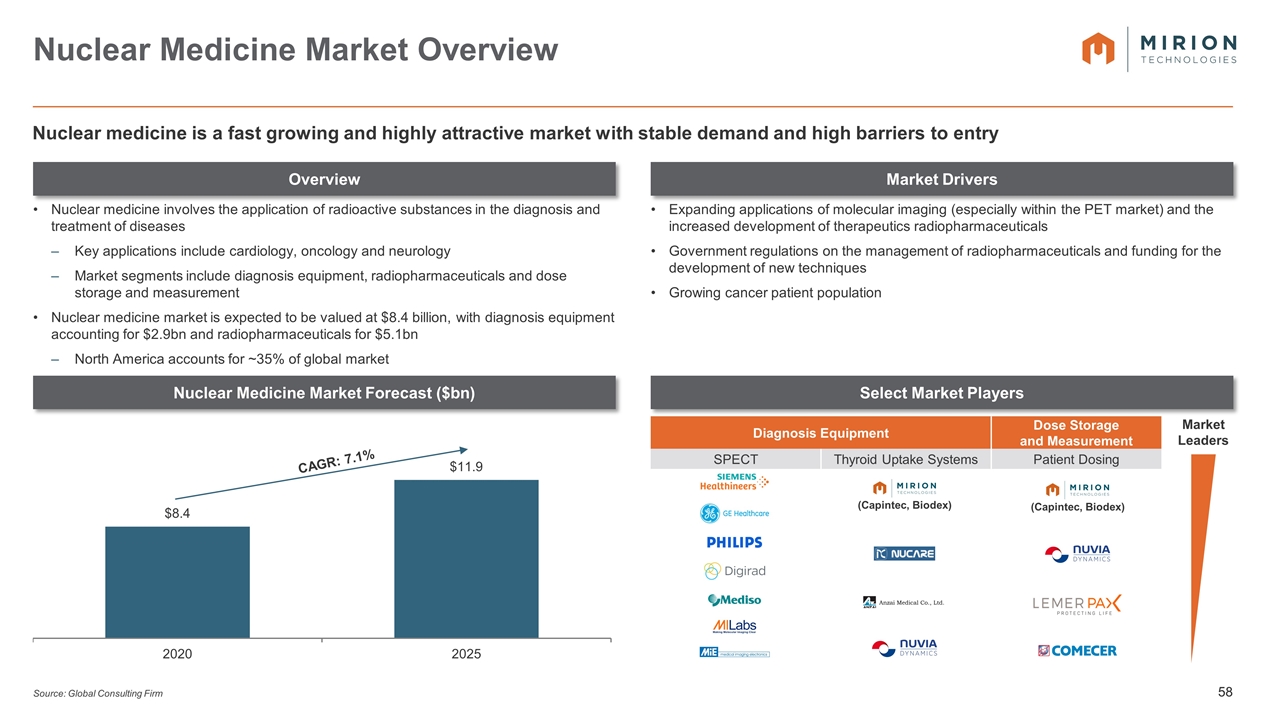
Diagnosis Equipment Dose Storage and Measurement SPECT Thyroid Uptake Systems Patient Dosing Source: Global Consulting Firm Nuclear Medicine Market Overview Overview Market Drivers Select Market Players Nuclear medicine is a fast growing and highly attractive market with stable demand and high barriers to entry Nuclear Medicine Market Forecast ($bn) Nuclear medicine involves the application of radioactive substances in the diagnosis and treatment of diseases Key applications include cardiology, oncology and neurology Market segments include diagnosis equipment, radiopharmaceuticals and dose storage and measurement Nuclear medicine market is expected to be valued at $8.4 billion, with diagnosis equipment accounting for $2.9bn and radiopharmaceuticals for $5.1bn North America accounts for ~35% of global market Expanding applications of molecular imaging (especially within the PET market) and the increased development of therapeutics radiopharmaceuticals Government regulations on the management of radiopharmaceuticals and funding for the development of new techniques Growing cancer patient population CAGR: 7.1% Market Leaders (Capintec, Biodex) (Capintec, Biodex)

Space & Big Science Overview
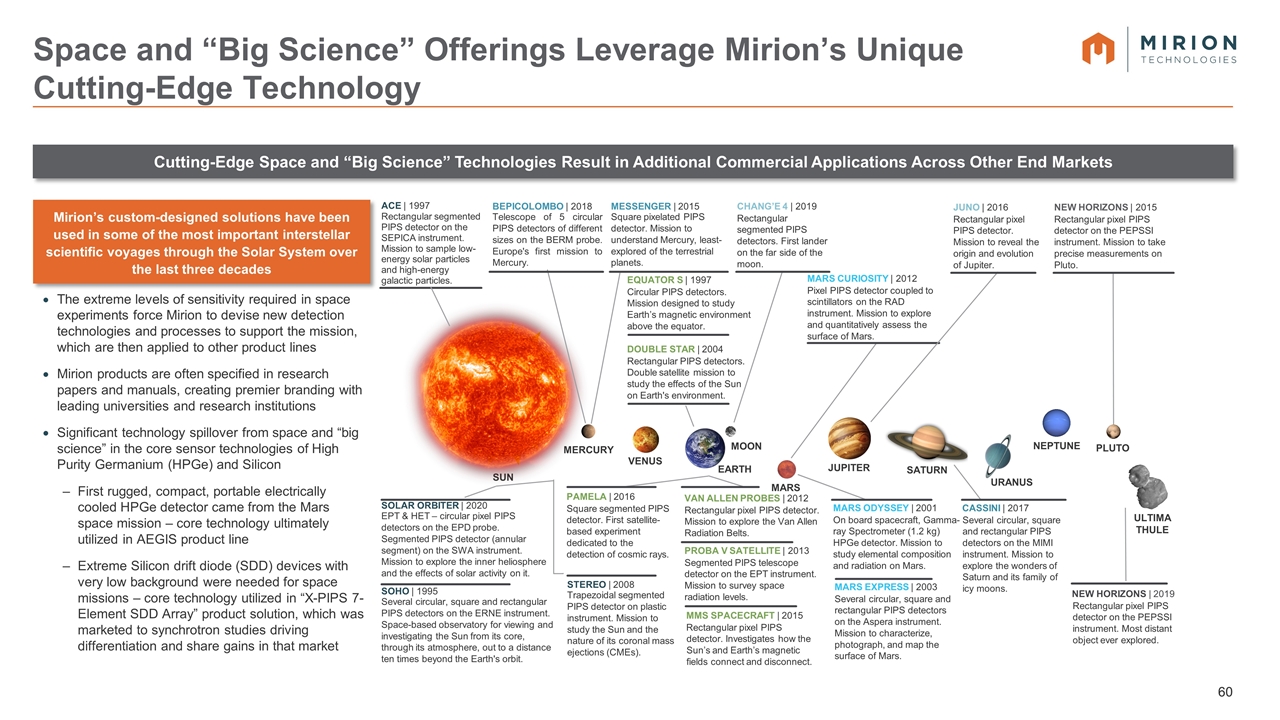
Space and “Big Science” Offerings Leverage Mirion’s Unique Cutting-Edge Technology The extreme levels of sensitivity required in space experiments force Mirion to devise new detection technologies and processes to support the mission, which are then applied to other product lines Mirion products are often specified in research papers and manuals, creating premier branding with leading universities and research institutions Significant technology spillover from space and “big science” in the core sensor technologies of High Purity Germanium (HPGe) and Silicon First rugged, compact, portable electrically cooled HPGe detector came from the Mars space mission – core technology ultimately utilized in AEGIS product line Extreme Silicon drift diode (SDD) devices with very low background were needed for space missions – core technology utilized in “X-PIPS 7-Element SDD Array” product solution, which was marketed to synchrotron studies driving differentiation and share gains in that market Mirion’s custom-designed solutions have been used in some of the most important interstellar scientific voyages through the Solar System over the last three decades ACE | 1997 Rectangular segmented PIPS detector on the SEPICA instrument. Mission to sample low-energy solar particles and high-energy galactic particles. BEPICOLOMBO | 2018 Telescope of 5 circular PIPS detectors of different sizes on the BERM probe. Europe's first mission to Mercury. MESSENGER | 2015 Square pixelated PIPS detector. Mission to understand Mercury, least-explored of the terrestrial planets. SOLAR ORBITER | 2020 EPT & HET – circular pixel PIPS detectors on the EPD probe. Segmented PIPS detector (annular segment) on the SWA instrument. Mission to explore the inner heliosphere and the effects of solar activity on it. STEREO | 2008 Trapezoidal segmented PIPS detector on plastic instrument. Mission to study the Sun and the nature of its coronal mass ejections (CMEs). PAMELA | 2016 Square segmented PIPS detector. First satellite-based experiment dedicated to the detection of cosmic rays. DOUBLE STAR | 2004 Rectangular PIPS detectors. Double satellite mission to study the effects of the Sun on Earth's environment. EQUATOR S | 1997 Circular PIPS detectors. Mission designed to study Earth’s magnetic environment above the equator. CHANG’E 4 | 2019 Rectangular segmented PIPS detectors. First lander on the far side of the moon. MARS CURIOSITY | 2012 Pixel PIPS detector coupled to scintillators on the RAD instrument. Mission to explore and quantitatively assess the surface of Mars. MARS EXPRESS | 2003 Several circular, square and rectangular PIPS detectors on the Aspera instrument. Mission to characterize, photograph, and map the surface of Mars. MARS ODYSSEY | 2001 On board spacecraft, Gamma-ray Spectrometer (1.2 kg) HPGe detector. Mission to study elemental composition and radiation on Mars. VAN ALLEN PROBES | 2012 Rectangular pixel PIPS detector. Mission to explore the Van Allen Radiation Belts. JUNO | 2016 Rectangular pixel PIPS detector. Mission to reveal the origin and evolution of Jupiter. NEW HORIZONS | 2015 Rectangular pixel PIPS detector on the PEPSSI instrument. Mission to take precise measurements on Pluto. CASSINI | 2017 Several circular, square and rectangular PIPS detectors on the MIMI instrument. Mission to explore the wonders of Saturn and its family of icy moons. NEW HORIZONS | 2019 Rectangular pixel PIPS detector on the PEPSSI instrument. Most distant object ever explored. PROBA V SATELLITE | 2013 Segmented PIPS telescope detector on the EPT instrument. Mission to survey space radiation levels. MMS SPACECRAFT | 2015 Rectangular pixel PIPS detector. Investigates how the Sun’s and Earth’s magnetic fields connect and disconnect. SUN MERCURY VENUS EARTH MOON MARS JUPITER SATURN URANUS NEPTUNE PLUTO ULTIMA THULE SOHO | 1995 Several circular, square and rectangular PIPS detectors on the ERNE instrument. Space-based observatory for viewing and investigating the Sun from its core, through its atmosphere, out to a distance ten times beyond the Earth's orbit. Cutting-Edge Space and “Big Science” Technologies Result in Additional Commercial Applications Across Other End Markets

Risk Factors and Additional Disclosures
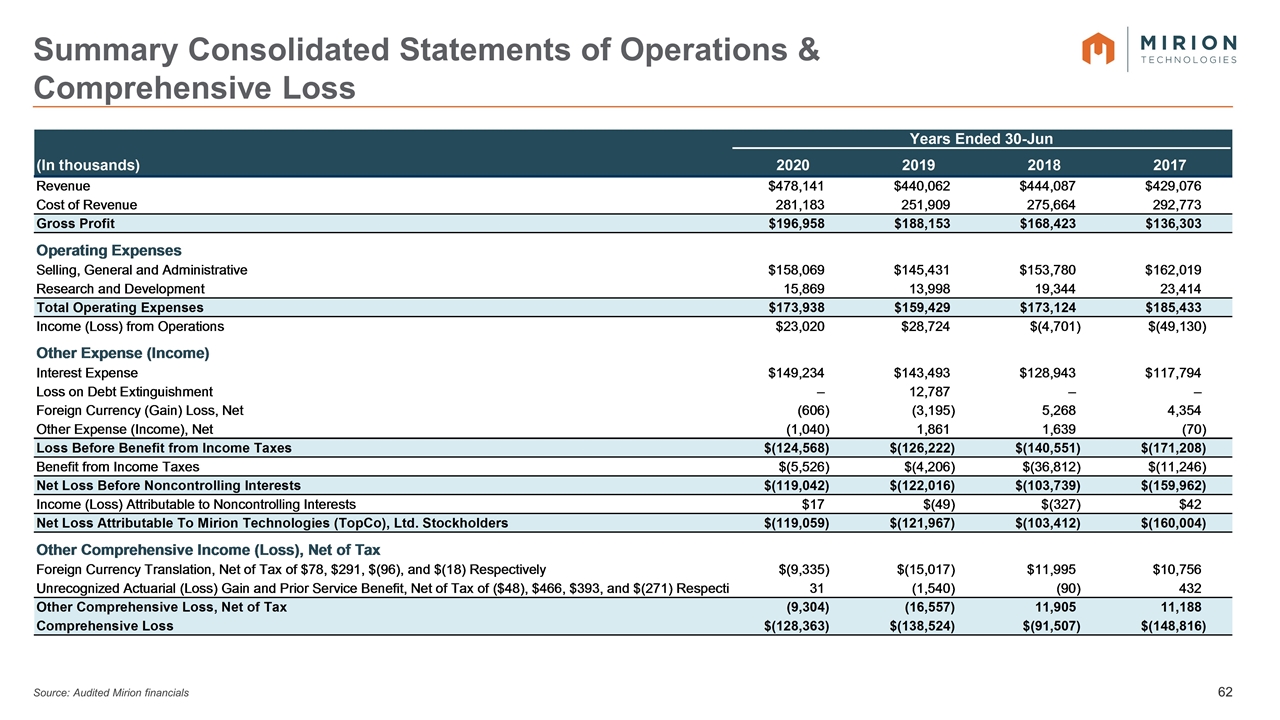
Summary Consolidated Statements of Operations & Comprehensive Loss Source: Audited Mirion financials
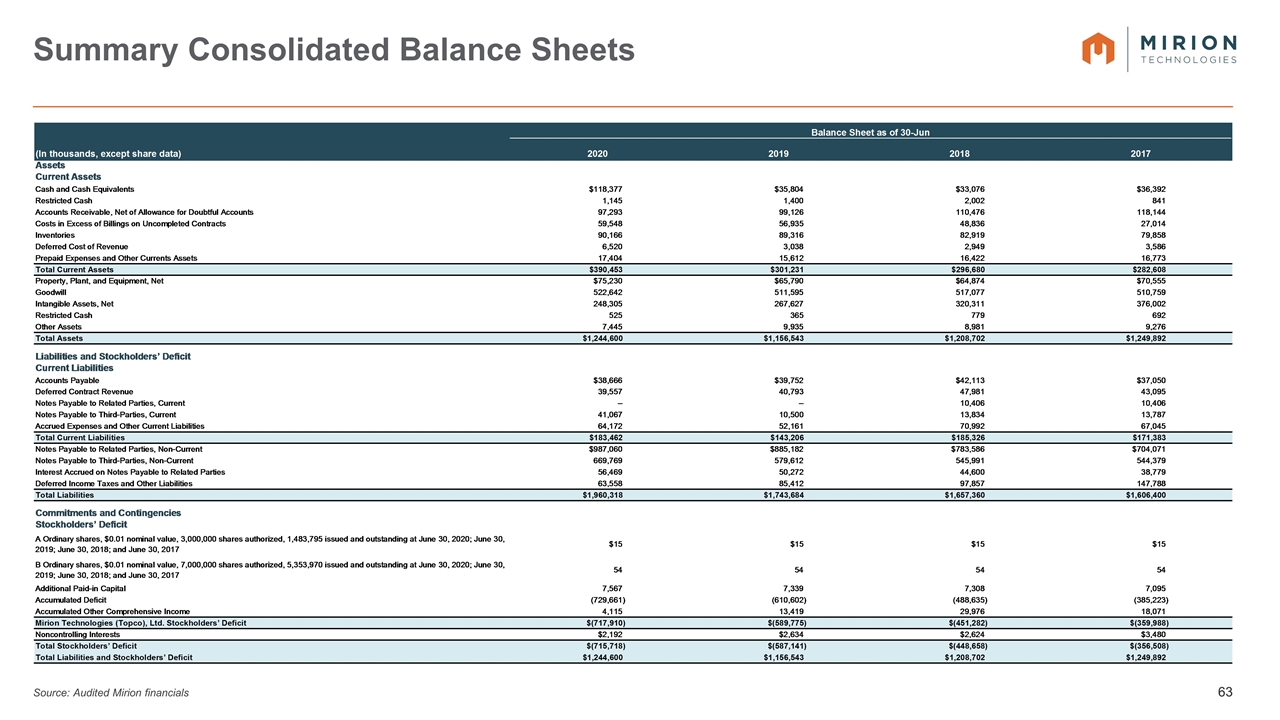
Summary Consolidated Balance Sheets Source: Audited Mirion financials
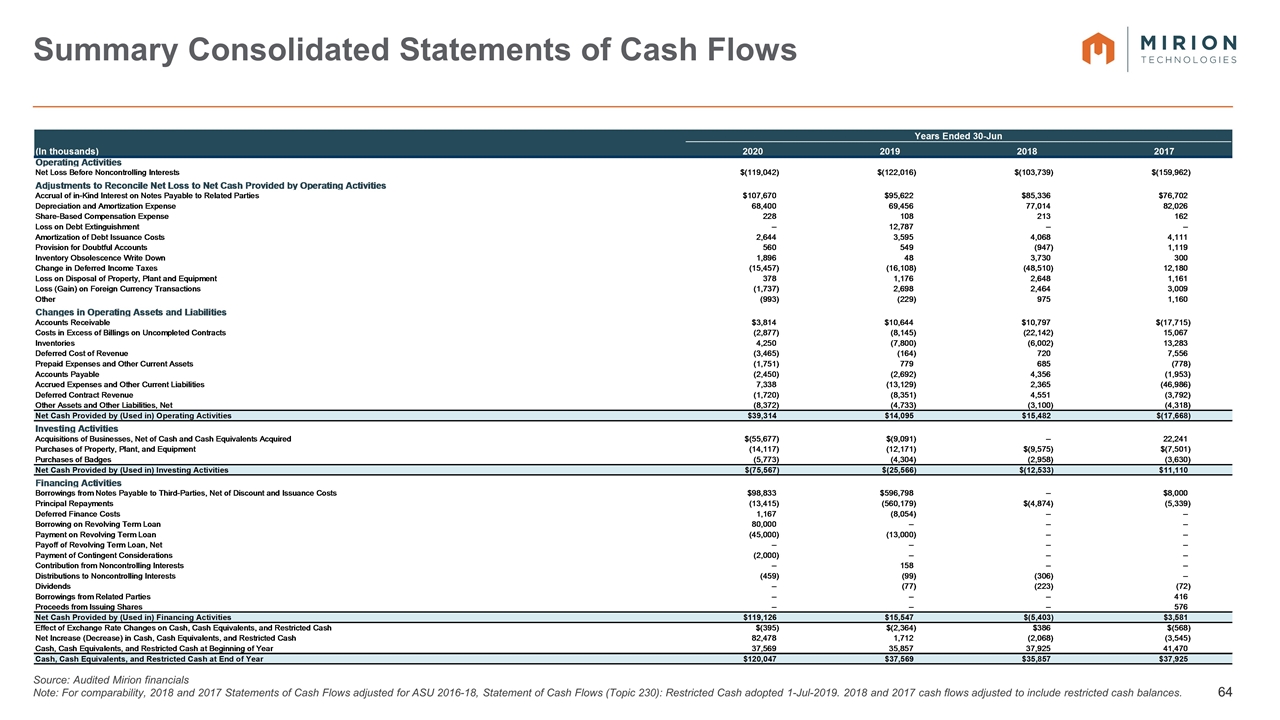
Summary Consolidated Statements of Cash Flows Source: Audited Mirion financials Note: For comparability, 2018 and 2017 Statements of Cash Flows adjusted for ASU 2016-18, Statement of Cash Flows (Topic 230): Restricted Cash adopted 1-Jul-2019. 2018 and 2017 cash flows adjusted to include restricted cash balances.
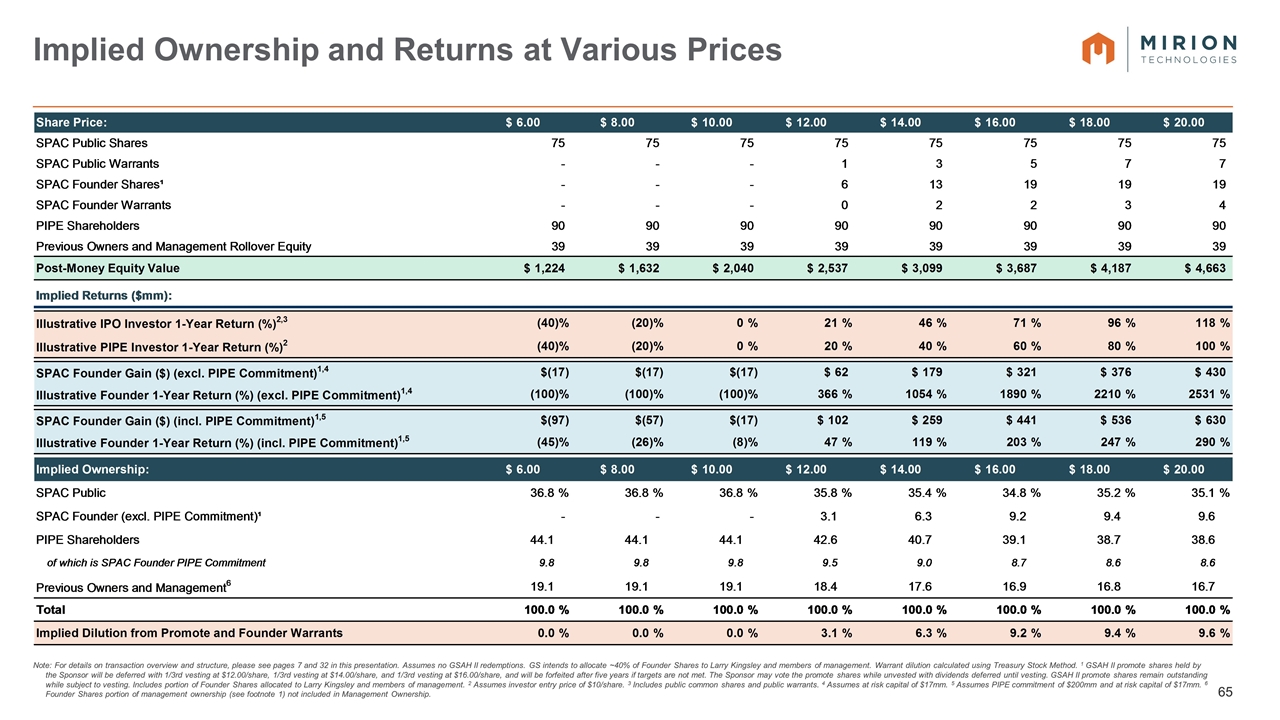
Note: For details on transaction overview and structure, please see pages 7 and 32 in this presentation. Assumes no GSAH II redemptions. GS intends to allocate ~40% of Founder Shares to Larry Kingsley and members of management. Warrant dilution calculated using Treasury Stock Method. 1 GSAH II promote shares held by the Sponsor will be deferred with 1/3rd vesting at $12.00/share, 1/3rd vesting at $14.00/share, and 1/3rd vesting at $16.00/share, and will be forfeited after five years if targets are not met. The Sponsor may vote the promote shares while unvested with dividends deferred until vesting. GSAH II promote shares remain outstanding while subject to vesting. Includes portion of Founder Shares allocated to Larry Kingsley and members of management. 2 Assumes investor entry price of $10/share. 3 Includes public common shares and public warrants. 4 Assumes at risk capital of $17mm. 5 Assumes PIPE commitment of $200mm and at risk capital of $17mm. 6 Founder Shares portion of management ownership (see footnote 1) not included in Management Ownership. Implied Ownership and Returns at Various Prices

Risk Factors Risks Related to Our Business and Industry Our global operations expose us to risks associated with public health crises and epidemics/pandemics, such as COVID-19. The global spread of COVID-19 has created significant volatility, uncertainty and worldwide economic disruption, resulting in an economic slowdown of potentially extended duration. We have incurred operating losses in the past and expect to incur operating losses in the future. Our financial performance may be variable. If we are unable to develop new products or enhance existing products to meet our customers’ needs and compete favorably in the market, we may be unable to attract or retain customers. We operate in highly competitive markets and in some cases compete against larger companies with greater financial resources. Our customers may reduce or halt their spending on our products and services. Our sales cycles in certain end markets can be long and unpredictable. Our growth plans depend in part on growth through acquisitions, and these plans involve numerous risks. If we are unable to make acquisitions, or if we are not successful in integrating the technologies, operations and personnel of acquired businesses or fail to realize the anticipated benefits of an acquisition, our operations may be materially and adversely affected. Many of our products and services involve the detection, identification, measurement or monitoring of radiation and the failure of our products or services to perform to specification could materially and adversely affect our business, financial condition or results of operations. Certain of our products require the use of radioactive sources or incorporate radioactive materials, which subjects us and our customers to regulations, related costs and delays and potential liabilities for injuries or violation of environmental, health and safety laws. We and many of our customers operate in a politically sensitive environment, and the public perception of nuclear energy or radiation therapy can affect our customers and us. Accidents involving nuclear power facilities, including but not limited to events similar to Fukushima, or terrorist acts or other high profile events involving radioactive materials could materially and adversely affect our customers and the markets in which we operate and increase regulatory requirements and costs that could materially and adversely affect our business. We have, and we intend to continue pursuing, fixed-price contracts. Our failure to mitigate certain risks associated with such contracts may result in reduced margins. We may not realize all of the sales expected from our backlog of orders and contracts, and amounts included in our order backlog may not result in actual revenue or translate into profits. Risks Related to Our Business Operations We operate as an entrepreneurial, decentralized company, which presents both benefits and certain risks. In particular, significant growth in a decentralized operating model may put strain on certain business group resources and our corporate functions, which could materially and adversely affect our business, financial condition and results of operations. A failure to expand our manufacturing capacity and scale our capabilities to manufacture new products could constrain our ability to grow our business. We rely on third-party manufacturers to produce non-core components for certain of our products and services. If our manufacturers are unable to meet our requirements, or are subject to unanticipated disruptions, our business could be harmed. We derive a significant portion of our revenue from international sales and our operations in foreign countries are subject to political, economic, legal and other risks, which could materially and adversely affect us. We rely on third-party sales representatives to assist in selling our products and services, and the failure of these representatives to perform as expected or to secure regulatory approvals in jurisdictions where they are required to do so could reduce our future sales. If our suppliers experience supply shortages and prices of commodities or components that we use in our operations increase, our results of operations could be materially and adversely affected. Our reliance upon sole or limited sources of supply for certain materials or components could cause production interruptions, delays and inefficiencies. Because we compete directly with certain of our customers and suppliers, our results of operations could be materially and adversely affected in the short term if these customers or suppliers abruptly discontinue or significantly modify their relationship with us. We derive a portion of our revenue from contracts with governmental customers or their contractors. Such customers are subject to increased pressures to reduce expenses. Government-funded contracts may also contain unusual or more onerous terms and conditions that are not common among commercial customers or risk subjecting us to audits, investigations, sanctions and penalties. Any reduction in the capital resources or government funding of our customers could reduce our sales and impede our ability to generate revenue. Many of our large contracts have penalties for late deliveries. A failure or breach of our or our vendors’ information technology, or IT, data security infrastructure, or the security infrastructure of our products, or the discovery or exploitation of defects or vulnerabilities in the same, may subject us and our products to increased vulnerability to unauthorized access and cyberattacks and could materially and adversely impact our or our customers’ business, financial condition, reputation and operations.

Risk Factors Risks Related to Our Business Operations (Cont’d.) Failure to secure and protect our trade secrets or other confidential or proprietary information from disclosure or misappropriation could materially and adversely affect our business, competitiveness and financial condition. Our future success is dependent on our ability to retain key personnel, including our executive officers, and attract qualified personnel. If we lose the services of these individuals or are unable to attract new talent, our business will be materially and adversely affected. If we encounter manufacturing problems, or if our manufacturing facilities do not continue to meet federal, state or foreign manufacturing standards, we may be required to temporarily cease all or part of our manufacturing operations, which would result in delays and lost revenue. Our customers’ localization requirements, in particular in China, India and South Korea, could materially and adversely affect our business. Our operations, and the operations of our suppliers, distributors or customers, could be subject to natural and manmade disasters and other business disruptions, which could materially and adversely affect our business and increase our expenses. Legal and Regulatory Risks We are subject to, or may otherwise be impacted by, a variety of federal, state, local and foreign laws and regulatory regimes. Failure to comply with such laws and regulations could subject us to, among other things, penalties and legal expenses which could have a material and adverse effect on our business, or such laws and regulations could otherwise impact us, directly or indirectly, in a manner that has a material and adverse effect on our business. We and our customers operate in highly regulated industries that require us and them to obtain, and comply with, federal, state, local and foreign government permits and approvals. Changes in industry standards and governmental regulations may increase our expenses or reduce demand for our products or services. We are subject to risks related to legal claims and proceedings filed by or against us, and adverse outcomes in these matters may materially harm our business. The Securities and Exchange Commission (“SEC”) has recently issued guidance on the accounting treatment of warrants. Such guidance may require us to restate or revise our financial statements, make new SEC filings or file amendments to existing filings or amend certain provisions of our warrant agreement. The application of this guidance may also result in a determination that we have a material weakness in our internal control over financial reporting. Legal and Regulatory Risks (Cont’d.) Legal, political and economic uncertainty surrounding the exit of the United Kingdom from the European Union, or Brexit, and the implementation of the trade and cooperation agreement between the United Kingdom and the European Union could materially and adversely affect our business. Enhanced international tariffs, including tariffs that affect our products or components within our products, other trade barriers or global trade wars or domestic preferences could increase our costs and materially and adversely affect our business operations and financial condition. We must comply with the U.S. Foreign Corrupt Practices Act, or FCPA, and analogous non-U.S. anti-bribery statutes including the UK Anti-Bribery Act. Our or our sales representatives’ failure to comply with such laws could subject us to, among other things, penalties and legal expenses that could harm our reputation and materially and adversely affect our business, financial condition and results of operations. Legal compliance with import and export controls, as well as with sanctions, in the United States and other countries, is complex, and compliance restrictions and expenses could materially and adversely impact our revenue and supply chain. Any failure of our products offerings could subject us to substantial liability, including product liability claims and indemnification claims, for which we may not have adequate insurance coverage or could damage our reputation or the reputation of one or more of our brands. Any actual or perceived failure to comply with evolving data privacy and data security laws and regulations in the jurisdictions where we operate, both inside and outside of the United States, could lead to government enforcement actions (which could include civil or criminal penalties), private litigation or adverse publicity and could materially and adversely affect our business. Our ability to compete successfully and achieve future growth will depend on our ability to obtain, maintain, protect, defend and enforce our intellectual property and to operate without infringing, misappropriating or otherwise violating the intellectual property of others. We may need to defend ourselves against third-party claims that we are infringing, misappropriating or otherwise violating others’ intellectual property rights, which could divert management’s attention, cause us to incur significant costs and prevent us from selling or using the technology to which such rights relate. Our use of “open source” software could negatively affect our ability to sell our products and subject us to possible litigation. Our obligations to indemnify our customers for the infringement, misappropriation or other violation by our products of the intellectual property rights of others could require us to pay substantial damages and impose other costs and fees. We could incur substantial costs as a result of violations of, or liabilities under, environmental laws. We do not control our suppliers, customers or business partners, and facts or circumstances that may occur as a result of their actions or omissions could harm our reputation and sales.

Risk Factors Legal and Regulatory Risks (Cont’d.) Some of our workforce is represented by labor unions in the United States and by works councils and trade unions in the EU, and are covered by collective bargaining agreements in connection with such representations. Labor group representation may lead to work stoppages that could materially and adversely affect our business, including as a result of a failure to renegotiate a collective bargaining agreement. The elimination or any modification of the Price-Anderson Act’s indemnification authority could have adverse consequences for our business. Certain of our products and software are subject to ongoing regulatory oversight by the Food and Drug Administration, or FDA, or equivalent regulatory agencies in international markets and if we are not able to obtain or maintain the necessary regulatory approvals we may not be able to continue to market and sell such products which may materially and adversely affect our business. Modifications, upgrades and future products related to our products may require new FDA clearances or premarket approvals and similar licensing or approvals in international markets. Such modifications, or any defects in design, manufacture or labeling may require us to recall or cease marketing the affected products or software until approvals or clearances are obtained. We are subject to federal, state, local and international laws and regulations related to healthcare, the violation of which could result in substantial penalties and harm our business in the medical end market. Healthcare reform legislation could materially and adversely affect demand for our products, our revenue and our financial condition. If third-party payors do not provide sufficient coverage and reimbursement to healthcare providers or if there is a reduction in the number of patients with health insurance, demand for our products and our revenue could be materially and adversely affected. Some of our products depend on our ability to source data from third parties who could take steps to block our access to such data. Such blocking could limit the effectiveness of these products, increase our expenses or materially and adversely impact our business. Regulations related to “conflict minerals” may force us to incur additional expenses, may result in damage to our business reputation and may materially and adversely impact our ability to conduct our business. Risks Related to Our Liquidity and Capital Resources If we cannot generate sufficient operating cash flow and obtain external financing, we may be unable to make all of our planned capital expenditures and other expenses. Our indebtedness could impair our financial condition and harm our ability to operate our business. Despite our levels of indebtedness, we have the ability to incur more indebtedness. Incurring additional debt could further intensify the risks described above. Restrictive covenants in our 2019 Credit Agreement and any future debt agreements, could restrict our operating flexibility. Unfavorable currency exchange rate fluctuations could materially and adversely affect our financial results. Changes in our effective tax rate or adverse outcomes resulting from examination of our income tax returns could materially and adversely affect our results. Risks Related to Ownership of Our Common Stock Following the Business Combination and Operating as a Public Company The price of our common stock and warrants may be volatile and subject to wide fluctuations. We have and will continue to incur increased costs as a result of becoming a reporting company. Our internal control over financial reporting has not been assessed for compliance with the standards required by Section 404 of the Sarbanes-Oxley Act, and failure to achieve and maintain effective internal control over financial reporting in accordance with Section 404 of the Sarbanes-Oxley Act could materially and adversely affect us. Future resales of our common stock after the consummation of the Business Combination may cause the market price of our securities to drop significantly, even if our business is doing well. Warrants will become exercisable for our common stock, which would increase the number of shares eligible for future resale in the public market and result in dilution to our stockholders. If securities or industry analysts do not publish research or reports about our business, if they adversely change their recommendations regarding our stock or if our results of operations do not meet their expectations, our stock price and trading volume could decline. We may be subject to securities litigation, which is expensive and could divert management attention.

Risk Factors Risks Related to Our Liquidity and Capital Resources (Cont’d.) Upon consummation of the Business Combination, our parent company will be a holding company, its principal asset will be its ownership interest in Mirion Technologies (Topco), Ltd, and it will accordingly be dependent upon distributions from Mirion Technologies (Topco), Ltd to pay dividends, if any, taxes and other expenses. Some provisions of our organizational and governing documents may deter third parties from acquiring us and diminish the value of our common stock. We may be subject to certain ownership and voting power laws and regulations which may limit the ability of stockholders to acquire our common stock. Our organizational and governing documents may include provisions to comply with such laws and regulations. Our organizational and governing documents include forum selection clauses, which could discourage claims or limit stockholders’ ability to make a claim against us, our directors, officers, other employees or stockholders. We do not anticipate paying any cash dividends for the foreseeable future. Our parent company will qualify as an “emerging growth company” within the meaning of the Securities Act, and if it takes advantage of certain exemptions from disclosure requirements available to emerging growth companies, it could make our securities less attractive to investors and may make it more difficult to compare our performance to the performance of other public companies.